| |
|
|
The key points from the European Society of Cardiology (ESC) Guide
for the Diagnosis and Treatment of Acute and Chronic Heart Failure (HF)
from 2021 [1] are presented, as well as some views from the American
ACC / AHA Guidelines from 2022 [2]:
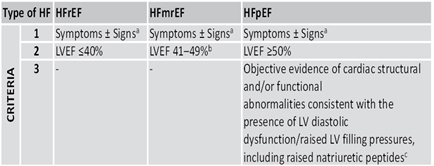
Heart failure (HF) nomenclature with left ventricular ejection
fraction (LVEF) of 41-49% has been revised in HF with mildly reduced
EF (HFmEF). HF with LVEF ≤40% remains HF with reduced EF (HFrEF),
and HF with LVEF ≥50% remains HF with preserved EF (HFpEF).
Table 1. Heart failure (HF) nomenclature from ESC guideline 2021
All patients with suspected HF should have: electrocardiogram,
transthoracic echocardiogram, X-ray of thorax (lung and heart),
complete blood count, urea, creatinine, electrolytes, thyroid
hormones, glycosylated hemoglobin (HbA1c), lipid status, iron
analysis, peptide (BNP / NT-proBNP). Magnetic resonance imaging of
the heart is recommended in patients with poor acoustic window for
ultrasound of the heart or in patients with suspected infiltrative
cardiomyopathy, amyloidosis , hemochromatosis, dilated
non-compaction cardiomyopathy or myocarditis [1]. The new diagnostic
algorithm for heart failure (HF) is shown in Figure 1.
FIGURE 1. DIAGNOSTIC ALGORITHM FOR HEART
INSUFFICIENCY (HF) ACCORDING TO THE NEW ESC GUIDE 2021.
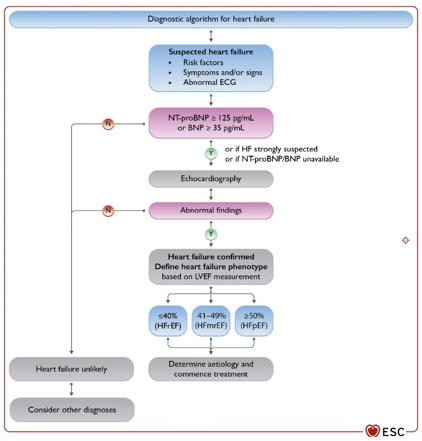
LEGEND: Heart failure with reduced left ventricular ejection
fraction (HFrEF)
Heart failure with mildly reduced left ventricular ejection fraction
(HFmrEF)
Heart failure with preserved left ventricular ejection fraction (HFpEF)
Available at www.escardio.org/guidelines (doi: 10.1093/eurheartj/ehab368)
Medical, primarily drug therapy directed by the New ESC Guide,
ie guidelines for patients with heart failure (HF) with reduced
ejection fraction (HFrEF) brings significant innovations and changes
in the treatment paradigm, from the gradual introduction of drugs to
the simultaneous introduction of 5 main classes of drugs.
Treatment of heart failure with reduced left ventricular ejection
fraction (HFrEF) and symptoms of class II-New York Heart Association
(NYHA) -dispnea at higher exertion and higher classes, now includes
angiotensin receptor inhibitor neprilysin (ARNI) as a substitute for
angiotenzin convertase enzyme inhibitor( ACEI). Another significant
innovation is the addition of SGLT-2 (Sodium Glucose channels
Cotransporter-2) inhibitors, dapagliflozin or empagliflozin in
first-line therapy for heart failure, simultaneously with the
introduction of beta-blockers, ACEI or ARNI, mineralocorticoid
receptor inhibitors and diuretics. class I. (picture 2)
Figure 2. Treatment of patients with HEART
INSUFFICIENCY WITH REDUCED EJECTION FRAGMENT (HFrEF) according to
the ESC guide from 2021
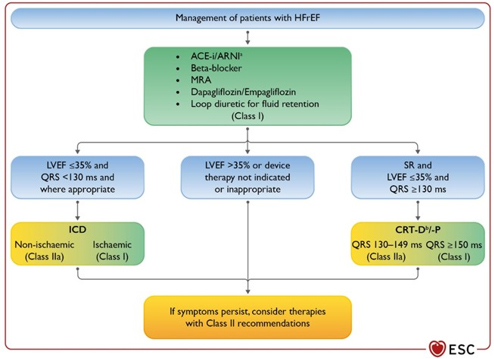
Legend ACE-I = angiotensin converting enzyme
inhibitor; ARNI = angiotensin receptor-neprilysin inhibitor; ARB =
angiotensin receptor blocker; BB = beta-blocker; CRT-D = pacemaker
for cardiac resynchronization with a defibrillator; CRT-P =
pacemaker for cardiac resynchronization; Available at
www.escardio.org/guidelines (doi: 10.1093/eurheartj/ehab368)
Excessive neurohumoral activation antagonists, beta-adrenergic
receptor blockers, and renin-angiotensin-aldosterone system
antagonists have shown a reduction in CV mortality in HFrEF in a
number of clinical randomized studies and have been the primary
therapy for heart failure for some time. These drugs achieved the
following beneficial effects: slowing the progression of left
ventricular remodeling, reducing discomfort, improving endurance and
quality of life in all symptomatic categories from NYHA class II to
NYHA class IV. Eplerenone as a selective mineralocorticoid
aldosterone receptor antagonist is recommended for NYHA class II,
while for severe class III-IV patients with beta-blockers and ACEIs
or sartans, a non-selective mineralocorticoid aldosterone receptor
antagonist beparon (beta blocker) should be added with . In
decompensated patients with severe congestion, Henle's loop
diuretics remain a pillar of therapy.
In the treatment of heart failure with reduced LVEF (HFrEF),
sacubitril-valsartan, a combined neprilysin and angiotensin
inhibitor (ARNI), was introduced in previous 2016 ESC guidelines,
which showed an additional reduction in CV mortality and
hospitalizations due to HFrEF compared to the ACE inhibitor enala .
Dapagliflozin and empagliflozin reduce the risk of cardiovascular
mortality or hospitalization due to HF in patients with HF and
reduced left ventricular ejection fraction <40% (HFrEF) [1] but
empagliflozin has also recently shown an effect in HFpEF [65%
ejection] .
In patients with HFrEF and NYHA class II to III symptoms, ARNi is
recommended to reduce morbidity and mortality (class 1A) [3-7].
In patients with previous or current symptoms of chronic HFrEF, the
use of ACEi is useful in reducing morbidity and mortality when ARNi
is not feasible (class 1A) [8-15].
In patients with previous or current symptoms of chronic HFrEF who
are intolerant to ACEi due to cough or angioedema and when the use
of ARNi is not feasible, the use of ARBs is recommended to reduce
morbidity and mortality [16-20].
In patients with previous or current symptoms of chronic HFrEF, in
whom the introduction of ARNi is not feasible, treatment with ACEi
or ARB gives high economic viability [2,21-27].
ARNi is contraindicated in concomitant ACEi or within 36 hours of
the last dose of ACEi, or in patients with a history of angioedema.
Recommendations for the administration of empagliflozin and
dapagliflozin that reduce cardiovascular mortality or
hospitalization due to HF in patients with HF and reduced left
ventricular ejection fraction <40% (HFrEF)
In patients with symptomatic chronic HFrEF, SGLT2i is recommended
to reduce hospitalization due to HF and cardiovascular mortality,
regardless of the presence of type 2 diabetes [28,29] and thus
introduced SGLT2i therapy has good economic justification [30,31].
Recommendations for HF with MILDLY reduced EF (HFmrEF)
In patients with HFmrEF, SGLT2i may be helpful in reducing
hospitalizations for HF and cardiovascular mortality [32]. Among
patients with current or previous symptomatic HFmrEF (LVEF, 41%
–49%), the use of ARNi, ACEi or ARB and MRA and evidence-based beta
blockers for HFrEF may be considered adequate for use to reduce the
risk of hospitalization for HF and cardiovascular mortality ,
especially among patients with LVEF at the lower end of this
spectrum [33-40].
Recommendations for HF with preserved EF (HFpEF) according to the
ACC / AHA guide from 2022 (ref 2)
- Patients with HFpEF and hypertension should be titrated with
antihypertensive drugs in order to achieve the target blood
pressure in accordance with published guidelines of clinical
practice for the prevention of morbidity [41-43].
- In patients with HFpEF, SGLT2 inhibitors may be useful in
reducing HF hospitalizations and cardiovascular mortality [44].
- In patients with HFpEF, treatment of atrial fibrillation
(AF) may be helpful in improving symptoms.
- In selected patients with HFpEF, mineralocorticoid receptor
(MRA) antagonists may be considered effective in reducing
hospitalizations, especially among patients with LVEF at the
lower end of this spectrum [45-47].
- In selected patients with HFpEF, the use of ARBs may be
considered to reduce hospitalizations, especially among patients
with LVEF at the lower end of this spectrum [48,49].
Implantable cardioverter-defibrillators (ICDs) are recommended
for the primary prevention of sudden cardiac death in symptomatic
ischemic or non-ischemic cardiomyopathy with LVEF ≤35% despite 3
months of optimal targeted therapy (GDMT) if 1-year survival is
expected. ICD is not recommended within 40 days of myocardial
infarction (MI) or for patients with NIHA class IV symptoms who are
not candidates for advanced therapy.
Cardiac pacemaker resinchronization (CRT) therapy is recommended for
symptomatic HFrEF with EF <35% in sinus rhythm with left bundle
branch block (LBBB) for 150 ms despite GDMT. It is also recommended
for HFrEF with EF <35% regardless of the symptoms or duration of
heart failure if there is a high degree of atrioventricular (AV)
block with the need for a pacemaker. (FIGURE 3)
FIGURE 3. Strategic review of care for patients
with heart failure and reduced left ventricular ejection fraction (HFrEF)
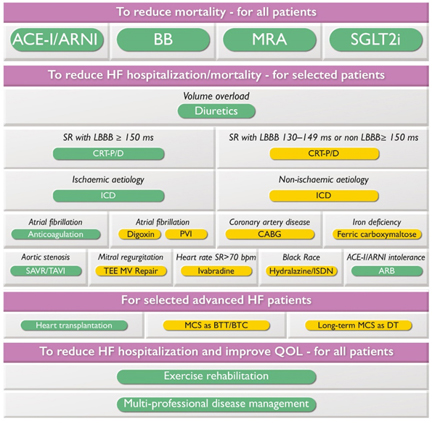
LEGEND: b.p.m = beats per minute; BTC = bridge to
transplant candidate; BTT = bridge to heart transplant; CABG =
surgical coronary artery bypass grafting; CRT-D = defibrillator
pacemaker resynchronization; CRT-P = pacemaker for cardiac
resynchronization; DT = definitive therapy; ICD = implantable
cardioverter-defibrillator; ISDN = isosorbide dinitrate; LBBB =
block of the left branch of the His bundle; MCS = mechanical
circulation support; MV = mitral valve; PVI = radiofrequency
isolation of pulmonary veins; SAVR = surgical replacement of the
aortic valve; SR = sinus rhythm; TAVI = transcatheter replacement of
the aortic valve; TEE MV repair = transcatheter MV reconstruction
from edge to edge.
Color code for recommendation class: green for recommendation class
I; Yellow for recommendation class IIa. The figure shows the
management options with Class I and IIa recommendations. See special
tables for those with Class IIb recommendations.
Available at www.escardio.org/guidelines (doi: 10.1093/eurheartj/ehab368)
For HFmEF, diuretics are recommended to alleviate or eliminate
congestion. ACE inhibitors / angiotensin receptor blockers / ARNI /
beta-blockers / mineralocorticoid receptor antagonists may be
considered as adjunctive therapy to reduce mortality and
hospitalization (Class IIa recommendation).
Diagnosis and treatment of factors that contribute to heart failure
(hypertension, kidney disease, etc.) and the use of diuretics are
recommended for patients with heart failure with preserved left
ventricular ejection fraction (HFpEF). Specific therapies have not
been shown to reduce mortality in HFpEF. However, after the release
of the ESC guide (August 2021), a new registration study
Emperor-preserved (2) appeared, where empagliflozin showed
improvement in the clinical outcome of treatment in patients with
heart failure and preserved LVEF> 40%. A pooled analysis of the
effects of empagliflozin 10 mg daily with pre-existing drug therapy
for heart failure was performed on 9,718 Emperor-reduced and
Emperor-Preserved patients. These two studies were comparable so
that a wide range of left ventricular ejection fraction from 25% to
65% was obtained. Studies have shown that empagliflozin reduces the
risk of hospitalization due to heart failure in a wide range of
ejection fraction values by up to 65%, and its efficiency is reduced
in patients with LVEF> 65%. There is also a beneficial effect of
empagliflozin on symptoms and endurance effort consistently with an
ejection fraction of less than 65%. Further analysis found that the
size of the therapeutic response to empagliflozin did not depend on
the size of LVEF in the range of 25% to 65%, with a similar
reduction in HF hospitalization risk to LVEF size in subgroups <30%
and 40-50%, and in the subgroup with preserved left ventricular
ejection fraction> 50%. An important fact from these studies is that
empagliflozin reduces the risk of worsening glomerular filtration (GFR)
in HF along the entire spectrum of the ejection fraction of LVEF,
both with reduced, slightly reduced and preserved LVEF from 25% to
65% (2).
For all patients with HF, enrollment in a multidisciplinary HF
program, at home or at the clinic, is recommended. For the
prevention of HF, Class I recommendations include: appropriate
hypertension treatment, statin use, when indicated, SGLT2 inhibitors
in diabetics at high risk for or with cardiovascular disease, and
counseling to discontinue, consume alcohol and drugs, and treat
obesity.
For acute decompensated HF, routine use of inotropic drugs is not
recommended in the absence of cardiogenic shock, and routine use of
opioid-morphine is also not recommended for cardiogenic pulmonary
edema. Routine use of an intra-aortic balloon pump in cardiogenic
shock after myocardial infarction is not recommended.
Additional Class I recommendations for hospitalized patients with
acute HF include the introduction of targeted oral therapy and the
careful elimination of pre-discharge volume overload (congestion)
with early follow-up within 1-2 weeks of hospital discharge.
For patients with atrial fibrillation (AF), routine use of
anticoagulants for CHA2DS2-VASc ≥2 in men and ≥3 in women is
recommended, preferably with direct-acting oral anticoagulants (NOAC),
except in the presence of a prosthetic mechanical valve or moderate
or severe mitral stenosis. Recommended. Emergency cardioversion is
recommended for patients with HF AF who are hemodynamically
compromised. Rhythm control, including radiofrequency catheter
ablation, should be considered in AF patients who have symptoms.
For patients with HF and severe aortic stenosis, transcatheter /
surgical replacement of the aortic valve using the Heart Time
approach is recommended. For patients with HF with secondary mitral
regurgitation, percutaneous edge-to-edge mitral valve repair should
be considered if severe symptoms persist despite appropriate guided
therapy (GDMT). For patients with secondary mitral regurgitation and
coronary artery disease requiring revascularization, coronary
by-pass and mitral valve surgery should be considered.
Patients with cancer who are being considered for cardiotoxic
chemotherapeutic drugs and who are at risk of cardiotoxicity should
ideally be evaluated by a cardio-oncologist before starting therapy.
Tafamidis is a Class I recommendation in patients with TTR-type
amyloidosis with symptoms of NIHA class I-II.
All patients with HF should be periodically examined for iron
deficiency anemia. Administration of ferric carboxymaltose should be
considered in symptomatic, outpatient patients with HF and anemia
due to iron deficiency and EF ≤45% or hospitalized patients with HF
with EF ≤50%.
REFERENCE:
- McDonagh TA, Metra M, Adamo M, et al.Citation:2021 ESC
Guidelines for the Diagnosis and Treatment of Acute and Chronic
Heart Failure: Developed by the Task Force for the Diagnosis and
Treatment of Acute and Chronic Heart Failure of the European
Society of Cardiology (ESC) With the Special Contribution of the
Heart Failure Association (HFA) of the ESC. Eur Heart J.
2021;42(36):3599-3726. doi: 10.1093/eurheartj/ehab368.
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ,
Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management
of Heart Failure. A Report of the American College of
Cardiology/American Heart Association Joint Committee on
Clinical Practice Guidelines. J Am Coll Cardiol.
2022;79(17):e263-e421. doi: 10.1016/j.jacc.2021.12.012. Epub
2022 Apr 1.
- McMurray JJ, Packer M, Desai AS, et al.
Angiotensinneprilysin inhibition versus enalapril in heart
failure. N Engl J Med. 2014;371:993–1004.
- Wachter R, Senni M, Belohlavek J, et al. Initiation of
sacubitril/valsartan in haemodynamically stabilised heart
failure patients in hospital or early after discharge: primary
results of the randomised TRANSITION study. Eur J Heart Fail.
2019;21:998–1007.
- Velazquez EJ, Morrow DA, DeVore AD, et al.
Angiotensin-neprilysin inhibition in acute decompensated heart
failure. N Engl J Med. 2019;380:539–548.
- Desai AS, Solomon SD, Shah AM, et al. Effect of
sacubitril-valsartan vs enalapril on aortic stiffness in
patients with heart failure and reduced ejection fraction: a
randomized clinical trial. JAMA. 2019;322:1077–1084.
- Wang Y, Zhou R, Lu C, et al. Effects of the
angiotensin-receptor neprilysin inhibitor on cardiac reverse
remodeling: meta-analysis. J Am Heart Assoc. 2019;8:e012272.
- Consensus Trial Study Group. Effects of enalapril on
mortality in severe congestive heart failure. Results of the
Cooperative North Scandinavian Enalapril Survival Study
(CONSENSUS). N Engl J Med. 1987;316:1429–1435.
- SOLVD Investigators. Effect of enalapril on survival in
patients with reduced left ventricular ejection fractions and
congestive heart failure. N Engl J Med. 1991;325:293–302.
- Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative
effects of low and high doses of the angiotensin-converting
enzyme inhibitor, lisinopril, on morbidity and mortality in
chronic heart failure. ATLAS Study Group. Circulation.
1999;100:2312–2318.
- Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril
on mortality and morbidity in patients with left ventricular
dysfunction after myocardial infarction: results of the Survival
and Ventricular Enlargement Trial. The SAVE Investigators. N
Engl J Med. 1992;327:669–677.
- Effect of ramipril on mortality and morbidity of survivors
of acute myocardial infarction with clinical evidence of heart
failure. The Acute Infarction Ramipril Efficacy (AIRE) Study
Investigators. Lancet. 1993;342:821–828.
- Køber L, Torp-Pedersen C, Carlsen JE, et al. A clinical
trial of the angiotensin-converting-enzyme inhibitor
trandolapril in patients with left ventricular dysfunction after
myocardial infarction. Trandolapril Cardiac Evaluation (TRACE)
Study Group. N Engl J Med. 1995;333:1670–1676.
- Garg R, Yusuf S. Overview of randomized trials of
angiotensin-converting enzyme inhibitors on mortality and
morbidity in patients with heart failure. Collaborative Group on
ACE Inhibitor Trials. JAMA. 1995;273:1450–1456.
- Woodard-Grice AV, Lucisano AC, Byrd JB, et al. Sex-dependent
and race-dependent association of XPNPEP2 C-2399A polymorphism
with angiotensinconverting enzyme inhibitor-associated
angioedema. Pharmacogenet Genomics. 2010;20:532–536.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial
Investigators. A randomized trial of the angiotensinreceptor
blocker valsartan in chronic heart failure. N Engl J Med.
2001;345:1667–1675.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan,
captopril, or both in myocardial infarction complicated by heart
failure, left ventricular dysfunction, or both [published
correction appears in N Engl J Med. 2004;350:203]. N Engl J Med.
2003;349:1893–1906.
- Konstam MA, Neaton JD, Dickstein K, et al, HEAAL
Investigators. Effects of high-dose versus low-dose losartan on
clinical outcomes in patients with heart failure (HEAAL study):
a randomised, double-blind trial. Lancet. 2009;374:1840–1848.
- ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan,
ramipril, or both in patients at high risk for vascular events.
N Engl J Med. 2008;358:1547–1559.
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant
subjects with cardiovascular Disease (TRANSCEND) Investigators,
Yusuf S, Teo K, et al. Effects of the angiotensin-receptor
blocker telmisartan on cardiovascular events in high-risk
patients intolerant to angiotensin-converting enzyme inhibitors:
a randomised controlled trial. Lancet. 2008;372:1174–1183.
- Banka G, Heidenreich PA, Fonarow GC. Incremental
cost-effectiveness of guideline-directed medical therapies for
heart failure. J Am Coll Cardiol. 2013;61:1440–1446.
- Dasbach EJ, Rich MW, Segal R, et al. The costeffectiveness
of losartan versus captopril in patients with symptomatic heart
failure. Cardiology. 1999;91:189–194.
- Glick H, Cook J, Kinosian B, et al. Costs and effects of
enalapril therapy in patients with symptomatic heart failure: an
economic analysis of the Studies of Left Ventricular Dysfunction
(SOLVD) Treatment Trial. J Card Fail. 1995;1:371–380.
- Paul SD, Kuntz KM, Eagle KA, et al. Costs and effectiveness
of angiotensin converting enzyme inhibition in patients with
congestive heart failure. Arch Intern Med. 1994;154:1143–1149.
- Reed SD, Friedman JY, Velazquez EJ, et al. Multinational
economic evaluation of valsartan in patients with chronic heart
failure: results from the Valsartan Heart Failure Trial
(Val-HeFT). Am Heart J. 2004;148:122–128.
- Shekelle P, Morton S, Atkinson S, et al. Pharmacologic
management of heart failure and left ventricular systolic
dysfunction: effect in female, black, and diabetic patients, and
cost-effectiveness. Evid Rep Technol Assess (Summ). 2003:1–6.
- Tsevat J, Duke D, Goldman L, et al. Cost-effectiveness of
captopril therapy after myocardial infarction. J Am Coll
Cardiol. 1995;26:914–919.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin
in patients with heart failure and reduced ejection fraction. N
Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303.
Epub 2019 Sep 19.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and
renal outcomes with empagliflozin in heart failure. N Engl J
Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. Epub
2020 Aug 28.
- Parizo JT, Goldhaber-Fiebert JD, Salomon JA, et al.
Cost-effectiveness of dapagliflozin for treatment of patients
with heart failure with reduced ejection fraction. JAMA Cardiol.
2021;6(8):926–935. doi: 10.1001/jamacardio.2021.1437.
- Isaza N, Calvachi P, Raber I, et al. Cost-effectiveness of
dapagliflozin for the treatment of heart failure with reduced
ejection fraction. JAMA Netw Open. 2021;4(7):e2114501. doi:
10.1001/jamanetworkopen.2021.14501.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in
heart failure with a preserved ejection fraction. N Engl J Med.
2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. Epub 2021
Aug 27.
- Cleland JGF, Bunting KV, Flather MD, et al. Betablockers for
heart failure with reduced, mid-range, and preserved ejection
fraction: an individual patient-level analysis of double-blind
randomized trials. Eur Heart J. 2018;39(1):26–35. doi:
10.1093/eurheartj/ehx564.
- Solomon SD, McMurray JJV, Anand IS, et al.
Angiotensin-neprilysin inhibition in heart failure with
preserved ejection fraction. N Engl J Med.
2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655. Epub 2019
Sep 1.
- Halliday BP, Wassall R, Lota AS, et al. Withdrawal of
pharmacological treatment for heart failure in patients with
recovered dilated cardiomyopathy (TRED-HF): an open-label,
pilot, randomised trial. Lancet. 2019; 393(10166):61-73. doi:
10.1016/S0140-6736(18)32484-X. Epub 2018 Nov 11.
- Nilsson BB, Lunde P, Grogaard HK, et al. Long-term results
of high-intensity exercise-based cardiac rehabilitation in
revascularized patients for symptomatic coronary artery disease.
Am J Cardiol. 2018;121(1):21–26. doi:
10.1016/j.amjcard.2017.09.011. Epub 2017 Oct 10.
- Solomon SD, Claggett B, Desai AS, et al. Influence of
ejection fraction on outcomes and efficacy of
sacubitril/valsartan (lcz696) in heart failure with reduced
ejection fraction: the Prospective Comparison of ARNI with ACEI
to Determine Impact on Global Mortality and Morbidity in Heart
Failure (PARADIGMHF) trial. Circ Heart Fail. 2016;9(3):e002744.
doi: 10.1161/CIRCHEARTFAILURE.115.002744.
- Tsuji K, Sakata Y, Nochioka K, et al. Characterization of
heart failure patients with mid-range left ventricular ejection
fraction-a report from the CHART-2 Study. Eur J Heart Fail.
2017;19(10):1258–1269. doi: 10.1002/ejhf.807. Epub 2017 Mar 31.
- Solomon SD, Vaduganathan M, Claggett BL, et al.
Sacubitril/valsartan across the spectrum of ejection fraction in
heart failure. Circulation. 2020;141(5):352–361. doi:
10.1161/CIRCULATIONAHA.119.044586. Epub 2019 Nov 17.
- Zheng SL, Chan FT, Nabeebaccus AA, et al. Drug treatment
effects on outcomes in heart failure with preserved ejection
fraction: a systematic review and meta-analysis. Heart.
2018;104(5):407–415. doi: 10.1136/heartjnl-2017-311652. Epub
2017 Aug 5.
- Thomopoulos C, Parati G, Zanchetti A. Effects of
bloodpressure-lowering treatment in hypertension: 9.
Discontinuations for adverse events attributed to different
classes of antihypertensive drugs: meta-analyses of randomized
trials. J Hypertens. 2016;34(10):1921–1932. doi:
10.1097/HJH.0000000000001052.
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs
Standard Blood Pressure Control and Cardiovascular Disease
Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial.
JAMA. 2016;315(24):2673–2682. doi: 10.1001/jama.2016.7050.
- SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A
randomized trial of intensive versus standard blood-pressure
control. N Engl J Med. 2015;373(22):2103–2116. doi:
10.1056/NEJMoa1511939. Epub 2015 Nov 9.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in
heart failure with a preserved ejection fraction. N Engl J Med.
2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. Epub 2021
Aug 27.
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for
heart failure with preserved ejection fraction. N Engl J Med.
2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731.
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional
variation in patients and outcomes in the Treatment of Preserved
Cardiac Function Heart Failure With an Aldosterone Antagonist
(TOPCAT) trial. Circulation. 2015;131(1):34–42. doi:
10.1161/CIRCULATIONAHA.114.013255. Epub 2014 Nov 18.
- Solomon SD, Claggett B, Desai AS, et al. Influence of
Ejection Fraction on Outcomes and Efficacy of
Sacubitril/Valsartan (LCZ696) in Heart Failure with Reduced
Ejection Fraction: The Prospective Comparison of ARNI with ACEI
to Determine Impact on Global Mortality and Morbidity in Heart
Failure (PARADIGM-HF) Trial. Circ Heart Fail. 2016;9(3):e002744.
doi: 10.1161/CIRCHEARTFAILURE.115.002744.
- Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of
candesartan in patients with chronic heart failure and preserved
left-ventricular ejection fraction: the CHARM-Preserved Trial.
Lancet. 2003;362(9386):777–781. doi:
10.1016/S0140-6736(03)14285-7.
- Lund LH, Claggett B, Liu J, et al. Heart failure with
mid-range ejection fraction in CHARM: characteristics, outcomes
and effect of candesartan across the entire ejection fraction
spectrum. Eur J Heart Fail. 2018;20(8):1230–1239. doi:
10.1002/ejhf.1149. Epub 2018 Feb 12.
|
|
|
|




