| |
|
|
DEFINITION AND EPIDEMIOLOGY OF LYME DISEASE Lyme disease
(LD), or MORBUS LYME, is a chronic multisystem infectious disease in
humans, transmitted through the bite of an infected hard tick,
Ixodes ricinus, carrying one of the bacteria such as Borrelia
burgdorferi (Bb) (Figure 1), and less commonly other Borrelia
species: Borrelia garinii (Bg) and Borrelia afzelii (Ba).
It is a multisystem disease that can affect the skin, joints, heart,
and nervous system. Lyme disease is particularly known for its
frequent manifestation as the characteristic “erythema migrans”, a
bull’s-eye-shaped skin rash [1].

Figure 1. Borrelia burgdorferi magnified 400 x
Source:
https://upload.wikimedia.org/wikipedia/commons/f/f3/Borrelia_burgdorferi_%28CDC-PHIL_-6631%29_lores.jpg
When discussing the developmental stages of ticks, the starting
point is the egg, which the female (adult) lays in early spring.
From these eggs, larvae hatch in early summer. The larva takes its
first blood meal from the nearest available animal—most often
micromammals (forest and field rodents, hedgehogs, and others).
Humans can also occasionally be bitten by larvae, although this
occurs much less frequently compared to other developmental stages
of the tick.
After feeding, the larva molts (matures) into a nymph, which then
takes a second blood meal from the nearest host. For nymphs, these
hosts typically include hares, birds, deer, and occasionally humans
who spend time in tick habitats. Borrelia is transmitted through
tick saliva during feeding, usually after 48 hours or longer.
There are three theories regarding the transmission of Borrelia
burgdorferi (Bb) from the tick to the next host, with two being most
widely accepted. The first suggests that during the tick’s intense
blood-feeding phase, once it becomes engorged, it regurgitates part
of its intestinal contents (Bb resides in the tick’s midgut). The
second, less common but still recognized in the literature [1,2],
proposes that Bb migrates from the midgut to the salivary glands.
This theory implies a transmission mechanism similar to that of
mosquitoes transmitting Plasmodium. Advocates of this theory often
recommend prophylactic antibiotic treatment after every tick bite,
which is incorrect.
Experimental studies on the transmission of Borrelia from infected
ticks to mice have shown that infection rarely occurs within the
first 24 hours of tick attachment. The likelihood of infection
increases with the tick’s duration of attachment—particularly after
48 hours, and especially after 72 hours. Therefore, information
about the tick’s attachment time (less than 24 hours) is extremely
important for the prevention of Lyme disease. Prompt and proper
removal of the tick within the first few hours can be crucial,
especially if the tick is infected with Bb [3].
In Lyme disease, the reservoir represents the ecological niche—the
place within the host (tick, small rodent, etc.) where the pathogen
lives, persists as a species, and/or reproduces, usually without
harming its host.
In the case of Lyme disease, the tick can serve both as a reservoir
of Bb and as the source of infection (the one that directly
transmits Bb to a new host). Once infected, a tick can transmit Bb
throughout all its developmental stages—from larva to adult—and even
transovarially (from female to offspring). The vector, or carrier of
the pathogen, is Ixodes ricinus (Europe), Ixodes pacificus and
Ixodes scapularis (America), Ixodes persulcatus (Asia), etc., while
the causative agent belongs to the Borrelia burgdorferi genospecies
[4,5].
The term “Borrelia cycle” is translated as either the life cycle of
Borrelia or the enzootic cycle of Borrelia in English. It refers to
the complex life cycle of the Lyme disease bacterium (Borrelia
burgdorferi), which alternates between tick vectors and vertebrate
hosts. This enzootic cycle involves the transmission of the
bacterium from an infected tick to a host, and potentially back to
another tick (Figure 2) [6].
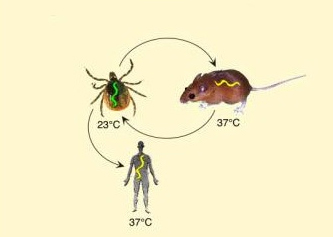
Figure 2. life cycle (transmission cycle) of
Borrelia, which alternates between the tick vector and the
vertebrate host.
Source:
https://upload.wikimedia.org/wikipedia/commons/0/08/Borrelia_cycle.jpg
CLINICAL ASPECTS OF LYME DISEASE
Lyme disease is the most common vector-borne infectious disease
in Europe and North America. LD is typically a seasonal illness,
occurring during periods of tick activity—from early spring and the
first warm days (nymphal stage), throughout June (larval and nymphal
stages), and up to the late autumn months (adult stage). During the
rest of the year, when tick activity ceases, Lyme disease does not
occur.
When searching for a diagnosis in patients presenting with symptoms
suggestive of LD, serological testing is most commonly used to raise
clinical suspicion. The incubation period ranges from 3 to 30 days,
from the tick bite to the appearance of signs and symptoms of Lyme
disease. It is important to note that not every erythema at the site
of a tick bite is Erythema migrans (EM). EM occurs in 60–80% of
cases and is often accompanied by flu-like symptoms.
At the site of the tick bite, within 5–7 days or longer, a
characteristic skin lesion may appear—Erythema migrans—which begins
as a macule or papule and can enlarge to as much as 50 cm in
diameter. EM presents as redness expanding from the bite site toward
the periphery in the form of irregular concentric rings with
serrated, more intensely red edges. The redness is flat, warm to the
touch (like surrounding skin), and does not cause pain or itching
[7,8,9].
This characteristic skin lesion—EM—is a hallmark sign of Lyme
disease. It differs from other skin rashes because it lacks tumor
(swelling) and dolor (pain), and the calor (warmth) is the same as
in surrounding skin [10]. Alongside the lesion (EM), early
symptoms—often flu-like—may include headache, mild fever (rare),
chills, shivering (rare), muscle and joint pain, lymphadenopathy,
and fatigue, which is profound, persistent, and unrelated to
physical activity [10,11]. Symptoms typically last around four weeks
[11].
In untreated patients, after several weeks, hematogenous
dissemination can occur, leading to systemic manifestations such as
fatigue, myalgia, and skin, cardiac, and neurological disorders
[10,11]. Arthritis develops in about 60% of patients, usually
monoarticular or oligoarticular, predominantly affecting the knee
joint—a sign of late-stage LD (third stage) [10,12].
Neurological manifestations occur in about 10–20% of patients, most
commonly facial nerve palsy. This belongs to the secondary stage and
is less common in Europe. The Centers for Disease Control and
Prevention (CDC) identifies this symptom as frequent in the United
States [12].
The second stage may also include carditis, occurring in
approximately 8% of untreated, infected individuals, presenting with
palpitations and atrioventricular (AV) conduction abnormalities, as
well as electrocardiographic changes in the S-T segment and T wave
[10,12].
The late stage, developing months or even years after untreated Lyme
disease, leads to polyarthritis and chronic skin lesions with
discoloration, known as acrodermatitis chronica atrophicans [11,13].
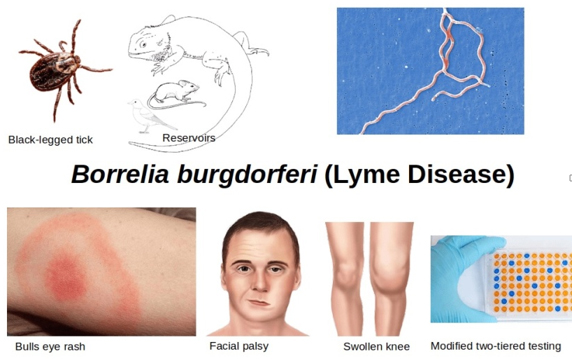
Figure 3. Borrelia Burgdorferi - Lyme disease
Source:
https://i0.wp.com/microbeonline.com/wp-content/uploads/2021/05/Borrelia-Burgdorferi-Lyme-Disease-min.png?ssl=1
Prolonged attachment of a tick to the skin increases the
likelihood of transmitting Borrelia burgdorferi. Therefore, timely
removal of the tick is crucial for reducing the risk of infection.
The longer the tick remains attached, the higher the probability of
pathogen transmission. For this reason, prompt and proper tick
removal is one of the most important steps in preventing the
clinical manifestation of Lyme disease [14,15].
If a tick is observed on the body, it is recommended to remove it as
soon as possible [16,17]. Ideally, this should be done in a
healthcare facility, where a physician can assess the risk of
infection and determine further management. If immediate
professional removal is not possible, the tick can be removed
independently using fine-tipped tweezers. The tweezers should grasp
the tick as close to the skin as possible, near its head, and pull
it out slowly, steadily, and evenly without sudden movements (Figure
4).
After removal, the bite site should be disinfected with alcohol or
iodine [18]. Regardless of successful removal, it is recommended to
see a physician promptly to evaluate the risk, monitor for potential
symptoms, and decide whether further diagnostic or prophylactic
measures are needed [17,19]. The key is not only proper tick removal
but also monitoring one’s health and seeking medical attention, as
infection can occur even after the tick has been removed [16,17].
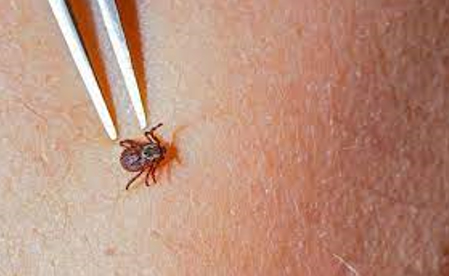
Figure 4. Removing ticks with tweezers
Source: https://www.bbc.com/serbian/lat/svet-69247310
Routine testing of ticks themselves for the presence of Borrelia
burgdorferi or other pathogens is not recommended for clinical
purposes, as a positive result does not confirm that infection has
been transmitted, nor does it determine therapeutic management. The
main criteria for deciding on prophylactic treatment include: the
tick species (Ixodes), endemic region, duration of attachment (>36
hours), and time since removal (<72 hours)—as recommended by the
IDSA/AAN/ACR Lyme disease guidelines (2020) [20].
LABORATORY DIAGNOSIS OF LYME DISEASE
The laboratory diagnosis of Lyme disease involves a combination
of methods applied according to the stage of the disease and its
clinical presentation [21,22]. The most accessible and widely used
diagnostic approach is serological testing of blood samples (ELISA,
confirmed by Western blot) [21,23]. In cases where neuroborreliosis
is suspected, both serological testing and PCR analysis of
cerebrospinal fluid (CSF) are performed [22,24].
The PCR method enables the direct detection of Borrelia burgdorferi
DNA in blood, CSF, or specific tissue samples; however, a negative
result does not exclude infection. Although removed ticks can also
be tested by PCR for pathogen detection, such testing has
epidemiological significance only and does not guide therapeutic
decisions [21,25].
Proper interpretation of laboratory findings requires integration of
test results with the clinical picture and epidemiological factors,
since serological tests may yield false-positive or false-negative
results, particularly in the early stages of the disease.
Algorithm of laboratory diagnosis of Lyme
disease
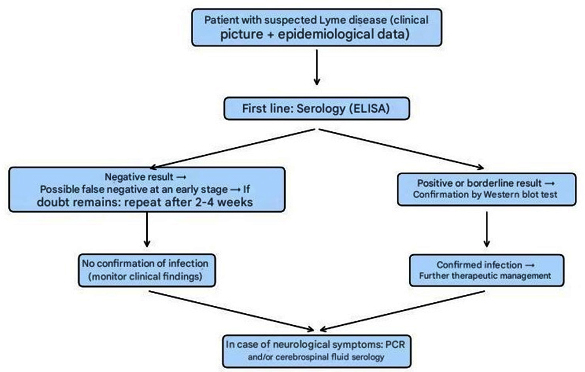
Table 1. Laboratory diagnostics
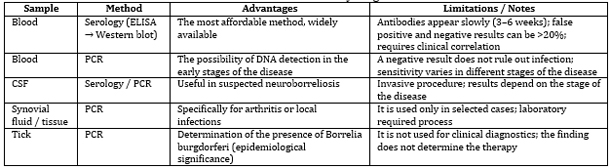
Ticks are not used for serological diagnosis in clinical
practice. Their testing serves exclusively epidemiological purposes
or research on the distribution of pathogens. Serological tests of
blood and cerebrospinal fluid remain the cornerstone of routine
laboratory diagnosis of Lyme disease.
Serological testing is the most accessible diagnostic approach
(performed by almost all public health institutes) and represents
the first step in the serological diagnosis of Lyme disease.
However, these tests are neither highly specific nor highly
sensitive, yielding more than 20% false-positive and false-negative
results. It is also important to emphasize that antibodies to
Borrelia burgdorferi develop slowly, and blood sampling should not
be performed before the end of the third or fourth week after the
onset of symptoms. Therefore, caution is required when interpreting
serological results in Lyme disease.
The main serological tests used for diagnosis are ELISA and
immunofluorescence assays (IFA). The ELISA test (Figure 5) for
Borrelia burgdorferi identifies the presence of IgM and IgG
antibodies, indicating whether it is an acute infection (IgM) or a
past infection (IgG)—although it is important to note that the
presence of IgG antibodies in Lyme disease does not always confirm a
past infection, as it might in other diseases [26,15].
IgM antibodies usually appear 2–4 weeks after the onset of the
erythema migrans lesion but are not always detectable at sufficient
levels for serological identification and typically disappear after
4–6 months. In some cases, IgM antibodies may persist for several
months after initial detection. IgG antibodies typically appear 8–12
weeks after the onset of illness and reach their peak within 4–6
months.
In the serological diagnosis of Lyme disease, the initial tests
include ELISA, EIA (enzyme immunoassay), or IFA (immunofluorescence
antibody assay). Negative results in the early phase of the disease
do not exclude the diagnosis, as antibodies may still be
insufficiently developed—particularly if antibiotic therapy was
initiated early or if erythema migrans is still present. Positive or
borderline results should be confirmed using the Western immunoblot
test (Figure 6).
If serological results are negative, but clinical symptoms of Lyme
disease persist, it is recommended to repeat testing after 2–4 weeks
[27,28].

Figure 5. How to choose the right propeller kit
Figure 6. Western blotting technique, used to
detect specific proteins in samples
Source: https://www.bmgrp.com/ Source: https://healthjade.net/western-blot/
how-to-choose-the-right-elisa-kit/
In addition to these tests, PCR is less commonly used in
diagnostics and is recommended for testing the tick itself for the
presence of Lyme disease pathogens (primarily for research purposes,
not routine diagnostics). PCR is also the only method used in
everyday diagnostics, apart from the cultivation of Borrelia on BSK
II medium, which is performed only in research settings.
False-positive serological results for Lyme disease can occur in
patients with syphilis. Early diagnosis and timely administration of
antimicrobial therapy play a key role in preventing cardiac,
neurological, and musculoskeletal complications. It is important to
note that antibiotics in the initial stage of Lyme disease do not
prevent the development of symptoms in later stages but serve as
prophylactic treatment to reduce the risk of disease progression.
TREATMENT OF LYME DISEASE
In the treatment of early Lyme disease, oral antibiotics such as
amoxicillin, doxycycline, or cefuroxime are used. The choice of
antibiotic and duration of therapy depend on the patient’s age and
the clinical stage of the disease. Doxycycline is generally avoided
in children under 8 years due to potential effects on teeth and
bones, while amoxicillin is preferred in pregnant women.
For patients with recurrent arthritis or involvement of the central
or peripheral nervous system, parenteral therapy with intravenous
antibiotics—most commonly ceftriaxone, cefotaxime, or penicillin
G—is administered. IV therapy is reserved for severe or chronic
forms of the disease, with dose, duration, and patient age being
crucial factors for treatment effectiveness and complication
prevention.
Lyme disease therapy is guided by the clinical form, disease
severity, and patient age. In early localized forms, oral
antibiotics—doxycycline, amoxicillin, or cefuroxime—are prescribed,
with restrictions for children under 8 years and pregnant women.
Treatment duration ranges from 10 to 21 days, depending on the
antibiotic and clinical presentation. For more severe or chronic
forms, including neuroborreliosis and recurrent arthritis,
parenteral therapy with intravenous ceftriaxone, cefotaxime, or
penicillin G is used, typically for 14–28 days. Treatment efficacy
depends on timely administration, appropriate dosage, therapy
duration, and patient age. [29,30].
Table 2. Lyme Disease Therapy: Antibiotics,
Dosages, and Duration
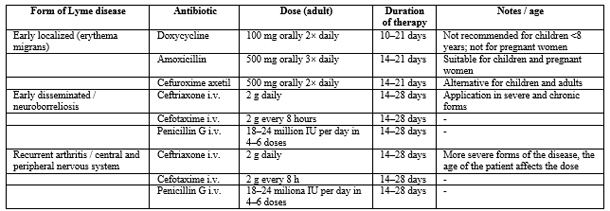
Notes: Doxycycline is not used in children under 8 years of age
and in pregnant women due to the risk to teeth and bones. Oral
therapy is applied in early localized forms. I.V. therapy is used in
severe, disseminated, or chronic forms, in cases of neuroborreliosis
and recurrent arthritis. The duration of therapy can be adjusted
according to the patient’s clinical response.
PREVENTION
Control of ticks in areas frequently visited by people (parks,
forested parks, recreational areas) represents a fundamental measure
for preventing tick bites and, consequently, reducing the risk of
Lyme disease transmission. Preventive activities can be divided into
ecological control measures, personal protective measures, and
public health interventions:
Ecological control measures include the application of appropriate
acaricides on limited areas with high tick populations, mowing and
maintenance of grassy areas—especially in places used for recreation
and play—removal of leaves, low vegetation, and branches in parks
and yards to reduce suitable tick habitats, control of rodent
populations that are natural reservoirs of Borrelia burgdorferi, and
minimizing human-rodent contact.
Personal protective measures involve wearing appropriate clothing
when in nature: long sleeves, long pants tucked into socks, closed
shoes, and light-colored clothing to facilitate tick detection;
using repellents based on DEET, icaridin, or permethrin (on
clothing), especially for individuals spending extended time
outdoors in endemic areas; and performing a full-body tick check
after outdoor activities, including hair, skin folds, and areas
where ticks commonly attach.
Public health interventions include educating the population about
the risks of tick bites, protection methods, and the importance of
early tick removal; organizing tick control campaigns in public
areas during peak activity seasons (spring and summer); monitoring
and surveillance of tick populations in endemic regions; and risk
mapping for the local population. [31-33].
CONCLUSION
Lyme disease represents a significant public health problem in
endemic areas of Europe and North America. Prevention is based on
reducing contact with ticks, wearing protective clothing, using
repellents, and implementing ecological measures to control tick and
rodent populations. Diagnosis is primarily clinical, supported by
serological testing, while molecular methods serve as supplementary
diagnostic tools. Timely and appropriate antibiotic therapy in the
early stage of the disease is crucial for preventing systemic
complications. Educating the public and healthcare personnel, as
well as proper tick removal, are the most effective strategies for
controlling and preventing Lyme disease.
LITERATURE:
- Connie R. Mahon I Donald C. Lehman TEXTBOOK OF DIAGNOSTIC
MICROBIOLOGY.ELSEVIER. 2019.
- Savić B, Mitrović S, Jovanović T. MEDICINSKA MIKROBIOLOGIJA.
Medicinski fakultet Beograd. 2022.
- Nikolić S., Stojanović D. INFEKTIVNE BOLESTI SA
EPIDEMIOLOGIJOM – priručnik za zdravstvene radnike, Nota,
Knjaževac, 1998 (ISBN 86.357.0437.1).
- Barbour, A. G., and G. R. Johnson. 1988. "The Borrelia
burgdorferi sensu lato: the Lyme disease spirochete." Annual
Review of Microbiology 42: 345-372.
- Stanek, G., et al. 2011. "Lyme borreliosis." The Lancet
379(9714): 461-473.
- Estrada-Peña A, de la Fuente J. The ecology of ticks and
epidemiology of tick-borne viral diseases. Antiviral Res.
2014;108:104-128. doi:10.1016/j.antiviral.2014.05.016.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis.
Lancet. 2012;379(9814):461-473.
doi:10.1016/S0140-6736(11)60103-7.
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW,
et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090.
doi:10.1038/nrdp.2016.90.
- Rizzoli A, Hauffe HC, Carpi G, Vourc’h GI, Neteler M, Rosa
R. Lyme borreliosis in Europe. Euro Surveill. 2011;16(27):19906.
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW,
et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090.
doi:10.1038/nrdp.2016.90.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis.
Lancet. 2012;379(9814):461-473.
doi:10.1016/S0140-6736(11)60103-7.
- Centers for Disease Control and Prevention (CDC). Lyme
Disease [Internet]. CDC; 2023. Available from:
https://www.cdc.gov/lyme
- Hu LT. Lyme disease. Ann Intern Med.
2016;164(9):ITC65-ITC80. doi:10.7326/AITC201605030
- Centers for Disease Control and Prevention (CDC). Lyme
Disease: Transmission [Internet]. Atlanta: CDC; 2024 [cited 2025
Oct 1]. Available from:
https://www.cdc.gov/lyme/causes/index.html
- European Centre for Disease Prevention and Control (ECDC).
Factsheet about Lyme borreliosis [Internet]. Stockholm: ECDC;
2025 [cited 2025 Oct 1]. Available from:
https://www.ecdc.europa.eu/en/lyme-borreliosis/facts
- Centers for Disease Control and Prevention (CDC). Tick
Removal and Testing [Internet]. CDC; 2023. Available from:
https://www.cdc.gov/ticks/removing_a_tick.html
- Stanek G, Strle F. Lyme borreliosis–from tick bite to
diagnosis and treatment. FEMS Microbiol Rev. 2018;42(3):233-258.
doi:10.1093/femsre/fux047.
- World Health Organization (WHO). Vector-borne diseases –
Ticks [Internet]. WHO; 2022. Available from:
https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere
AC, Klempner MS, et al. The clinical assessment, treatment, and
prevention of Lyme disease, human granulocytic anaplasmosis, and
babesiosis: clinical practice guidelines by the Infectious
Diseases Society of America. Clin Infect Dis.
2006;43(9):1089-1134. doi:10.1086/508667.
- Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT,
Aguero-Rosenfeld ME, Auwaerter PG, et al. Clinical Practice
Guidelines by the Infectious Diseases Society of America (IDSA),
American Academy of Neurology (AAN), and American College of
Rheumatology (ACR): 2020 Guidelines for the Prevention,
Diagnosis and Treatment of Lyme Disease. Clin Infect Dis.
2021;72(1):e1-e48.
- Centers for Disease Control and Prevention (CDC). Lyme
Disease: Laboratory Testing [Internet]. CDC; 2023. Available
from:
https://www.cdc.gov/lyme/diagnosistesting/labtest/twostep/index.html
- Stanek G, Strle F. Lyme borreliosis–from tick bite to
diagnosis and treatment. FEMS Microbiol Rev. 2018;42(3):233-258.
doi:10.1093/femsre/fux047.
- Hu LT. Lyme disease. Ann Intern Med.
2016;164(9):ITC65-ITC80. doi:10.7326/AITC201605030.
- Mygland Å, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard
E, Steiner I. EFNS guidelines on the diagnosis and management of
European Lyme neuroborreliosis. Eur J Neurol. 2010;17(1):8-16.
doi:10.1111/j.1468-1331.2009.02862.x.
- Dessau RB, van Dam AP, Fingerle V, Gray J, Hunfeld KP,
Jaulhac B, et al. To test or not to test? Laboratory support for
the diagnosis of Lyme borreliosis: a position paper of ESGBOR,
ESCMID Study Group for Lyme Borreliosis. Clin Microbiol Infect.
2018;24(2):118-124. doi:10.1016/j.cmi.2017.08.025.
- Infectious Diseases Society of America; American Academy of
Neurology; American College of Rheumatology. Guidelines for the
Prevention, Diagnosis and Treatment of Lyme Disease. Clin Infect
Dis. 2020;72(1):e1-e48. OUP Academic
- European Centre for Disease Prevention and Control (ECDC).
Factsheet about Borreliosis (Lyme disease) [Internet].
Stockholm: ECDC; 2025 [cited 2025 Oct 1]. Available from:
https://www.ecdc.europa.eu/en/borreliosis/facts/factsheet
Evropski centar za prevenciju bolesti
- Centers for Disease Control and Prevention (CDC). Clinical
Care of Lyme Disease [Internet]. Atlanta: CDC; 2024 [cited 2025
Oct 1]. Available from:
https://www.cdc.gov/lyme/hcp/clinical-care/index.html cdc.gov
- Lantos PM, et al. 2020 guidelines for the prevention,
diagnosis, and treatment of Lyme disease. Arthritis Care Res
(Hoboken). 2021 Jan;73(1):1-9. doi: 10.1002/acr.24495.
- Centers for Disease Control and Prevention (CDC). Lyme
disease treatment. Available from:
https://www.cdc.gov/lyme/treatment/index.html
- European Centre for Disease Prevention and Control (ECDC).
Personal protective measures against tick bites. Available from:
https://www.ecdc.europa.eu/en/disease-vectors/prevention-and-control/protective-measures-ticks
- National Park Service (NPS). Ticks and tickborne diseases.
Available from:
https://www.nps.gov/articles/000/ticks-and-tickborne-diseases.htm
- National Institute for Occupational Safety and Health
(NIOSH). Tickborne diseases in workers. Available from:
https://www.cdc.gov/niosh/outdoor-workers/about/tick-borne-diseases.html
|
|
|
|









