| |
|
|
INTRODUCTION The most common conditions that lead to acute
pain in gynecological practice include ectopic pregnancies, pelvic
inflammatory disease, ruptured ovarian cysts, ovarian torsion,
torsion and degeneration of uterine leiomyomas, and spontaneous
miscarriages. The largest number of ovarian torsions is seen in the
reproductive period, around 71% [2], but it also occurs in fetuses
and neonates, premenarchal girls, pregnant women, and postmenopausal
women. Although the true incidence of torsion is still unknown, data
show that torsion accounts for 2.7% of surgical interventions,
making it the fifth most common condition requiring emergency
surgery [2]. Another study found that 15% of surgically treated
adnexal masses are in torsion [3]. Timely diagnosis is important
both for preserving ovarian function and for preventing subsequent
comorbidities.
PATHOGENESIS AND RISK FACTORS
To understand how torsion occurs, we must first understand the
anatomy of the supporting structures of the uterus and ovaries. The
ovary is a paired intraperitoneal organ with two primary functions:
the production of sex hormones, and the development and release of
the oocyte during ovulation, as well as the formation of the corpus
luteum, which provides sufficient hormonal support to early
pregnancy until placental function is established. What is specific
and remarkable about the ovary is that it can increase its volume
several hundred times during a woman’s reproductive period without
pathological clinical manifestations [4].
The ovary is a mobile structure, suspended from the pelvic wall by
the infundibulopelvic ligament (also called the suspensory ligament
of the ovary), through which the ovarian artery passes, and attached
to the uterus by the utero-ovarian ligament (ligamentum ovarium
proprium), through which the ovarian branch of the uterine artery
passes. In addition to providing support, these ligaments also serve
a nutritive role, as the blood vessels supplying the ovary run
through them, ensuring dual vascularization of the ovary [5].
Torsion occurs as a result of partial or complete rotation of the
adnexal supporting structures, during which the ovary and fallopian
tube rotate around both the infundibulopelvic and utero-ovarian
ligaments, resulting in partial or complete obstruction of ovarian
blood flow [6,7,8]. The thin walls of the veins are more prone to
complete occlusion compared to the muscular walls of arterial
vessels. Continuous arterial inflow without venous outflow leads to
edema with visible ovarian enlargement. Further vascular compression
results in ovarian ischemia, leading to necrosis, local hemorrhage,
and loss of function [9]. Most often, both the ovary and the
fallopian tube undergo torsion simultaneously, although isolated
torsion of the ovary or tube may occur, referred to as partial
torsion. Torsion involving paraovarian or paratubal cysts has also
been described [6]. The right ovary is more frequently affected than
the left, possibly because the right utero-ovarian ligament is
longer, and the presence of the sigmoid colon prevents torsion on
the left side [8,10]. Bilateral asynchronous ovarian torsion is also
possible, though rare [11]. The severity of symptoms and
morphological ovarian changes depends on the type and degree of
vascular occlusion. Based on anatomical features and clinical
findings, we can define risk factors for adnexal torsion. Greater
ovarian mobility is associated with torsion in premenarchal girls,
who have elongated infundibulopelvic ligaments. In this population,
more than half of patients have morphologically normal ovaries.
After this premenarchal period, with puberty, the incidence of
ovarian torsion decreases due to shortening of the infundibulopelvic
ligaments. Risk factors in premenarchal girls may also include the
presence of functional cysts or benign tumors, most commonly
teratomas and cystadenomas [12,13,14].
Ovarian torsion has also been described in the fetal period
(ultrasound may monitor cyst growth and secondary changes such as
hemorrhage, calcifications, or resorption) and in neonates [15].
The highest percentage of ovarian torsions occurs in women of
reproductive age with adnexal changes such as functional ovarian
cysts and benign tumors [8,9,16]. Malignant tumors and endometriotic
cysts are rarely the cause of ovarian torsion. In case series, the
percentage of malignant ovarian tumors associated with torsion is
reported to be below 3%. This is because such lesions cause
peritoneal reactions and adhesions that fix the mass, thereby
limiting its mobility [17]. More than 80% of patients with ovarian
torsion have ovarian masses larger than 5 cm in diameter. The size
of the ovarian mass correlates with the risk of torsion. In a series
of 87 case studies, ovarian masses ranged widely from 3 to 30 cm,
with an average of about 9.5 cm [18]. About 10–22% of ovarian
torsions occur during pregnancy. The incidence is somewhat higher
between the 10th and 17th weeks of gestation in the presence of
ovarian masses larger than 4 cm. Ovulation, the corpus luteum, and
ovulation induction in infertility treatment may cause ovarian
hyperstimulation syndrome, with multiple large cystic ovarian
changes that are prone to torsion. Polycystic ovary syndrome is also
a risk factor [19]. On the other hand, in patients who have
undergone a surgical procedure, the incidence of ovarian torsion is
about 2–15%, typically due to strangulation of the ovarian pedicle
around an existing adhesion. Recurrent torsion has also been
described, and studies show that individuals who have experienced
ovarian torsion once are at increased risk of developing torsion
again—either of the same ovary (“salvage ovary”) or of the
contralateral ovary. [8].
Clinical presentation and clinical findings Ovarian torsion caused
by the presence of an adnexal mass results in a variety of symptoms,
clinical signs, and presentations. The most common symptom is acute,
sharp pain in the lower abdomen or pelvis, accompanied by nausea and
vomiting (70%) in women of reproductive age, in the presence of an
adnexal mass or enlarged ovaries in PCOS or ovarian hyperstimulation,
or in women with a history of prior ovarian torsion [17,18,20]. Some
patients experience only nausea without vomiting. Abdominal pain is
most often intermittent, colicky in nature, with gradual
intensification and relief, although it may also be continuous. The
pain arises secondarily due to occlusion of the vascular pedicle and
is refractory to analgesics. It may radiate to the inguinal region
or flank. Premenarchal patients may report diffuse abdominal pain,
as they often find it difficult to localize the discomfort. In this
group, vomiting is the most common symptom in the absence of adnexal
pathology—this represents a vagal reflex response due to peritoneal
irritation. In neonates, torsion may present with feeding
difficulties, abdominal distension, vomiting, and irritability.
Ovarian torsion without infectious pathology may also be accompanied
by low-grade fever. The low-grade fever is explained by necrotic
changes in the torted ovary and occurs in 2–20% of patients.
Physical examination may reveal low-grade fever, abdominal
tenderness, abdominal pain, and a pelvic adnexal mass. A further
diagnostic challenge is that 30% of patients—especially those in the
premenarchal period—may have neither abdominal pain nor abdominal
tenderness. [17,18,21-24].
Diagnosis
The diagnosis of ovarian torsion most often requires a combination
of anamnestic data, clinical examination, and imaging methods. The
first approach to the patient is the physical examination and taking
the medical history. Anamnestic data may indicate a recent diagnosis
of an adnexal mass, recurrent abdominal pain, and low-grade fever.
In children aged 2–14 years, with high sensitivity and positive
predictive value, the Bolli score can be applied. The Bolli score
includes only the patient’s clinical data but not imaging methods
and identifies three useful clinical variables on the basis of which
the ovarian torsion score is established: the age of the child, the
duration of pain, and vomiting. –Number of points – number of years,
minus three points if vomiting is present, plus one point if the
duration of pain is longer than 12 hours. The cut-off value of the
Bolli score in girls is 11.5, a lower score indicating a higher
probability that ovarian torsion is present. [25,26].
LABORATORY TESTING should include hematocrit, leukocyte
count, human chorionic gonadotropin (HCG), electrolytes, and
inflammation parameters—C-reactive protein (CRP) [2,22]. Laboratory
analyses may be completely normal, may indicate anemia in the case
of corpus luteum rupture, or leukocytosis and elevated CRP due to
tissue necrosis and consequent inflammation. The level of
interleukin 6 is also elevated and indicates increased oxidative
stress in torsion, but it is also a nonspecific sign of inflammation
and is not routinely performed in our clinical practice [27,28].
Determining tumor markers has not proven to be sufficiently
sensitive or specific, although the elevation of certain tumor
markers may indicate the nature of the torted adnexal mass. The
physical exam is focused on abdominal palpation in order to detect a
tumor mass and assess the presence of peritoneal irritation. Imaging
studies are the most important. Ultrasound is the first-line
diagnostic tool [29,30].
In the pediatric population, transabdominal ultrasound with a full
bladder is the initial imaging method for evaluating torsion. The
sensitivity of transabdominal ultrasound in the pediatric population
is 92–93%, with a specificity of 96–100%. In adult women,
transvaginal ultrasound shows excellent specificity but variable
sensitivity, ranging from 35–85% [31]. What is monitored on
ultrasound is ovarian volume, presence of edema, presence of an
adnexal mass, presence of free fluid, and color Doppler of ovarian
or tumor mass blood vessels. The presence of a difference in ovarian
volume with its displacement is a pathognomonic sign of ovarian
torsion. Another sign is the presence of edema of normal ovarian
tissue. In the literature, it is described as the presence of
peripheral follicles with hyperechogenic halos in the ovary without
cystic changes or tumors — strings of pearls. The presence of
ovarian edema should not be mistaken for the presence of a solid
ovarian tumor.
The torted ovary may be rounder and enlarged compared to the
contralateral one due to swelling of vascular and lymphatic vessels.
There may be normal, reduced, or completely absent blood flow
through the vessels of the torted ovary [31–34]. The whirlpool sign
is a highly sensitive and specific sign for the diagnosis of ovarian
torsion. The whirlpool sign indicates the twisted vascular pedicle,
and Doppler sonography reveals circular blood vessels within the
mass [32]. Finally, a small amount of free fluid may be present in
the pouch of Douglas [31]. The greatest diagnostic challenge is
torsion without twisting of the ipsilateral ovary. It has been shown
that 31% of all torsions are incomplete adnexal torsions. A useful
sign of torsion involving only the tube but not the ovary is the
presence of three or more cysts in one row [35]. The combination of
free fluid in the pelvis, an enlarged ovary, and vascular
abnormalities increases the sensitivity and specificity of
ultrasound findings. CT of the abdomen and pelvis shows high
sensitivity and specificity in the evaluation of suspected torsion
[36] and may show an enlarged ovary, its displacement and pulling of
the uterus to that side, thickening of the cystic mass, ascites,
thickened walls of the tube [36,37]. The definitive diagnosis is
made in the operating room by direct visualization of the specimen.
Algorithm 1. Algorithm for management in cases of
clinical and imaging suspicion of ovarian torsion.
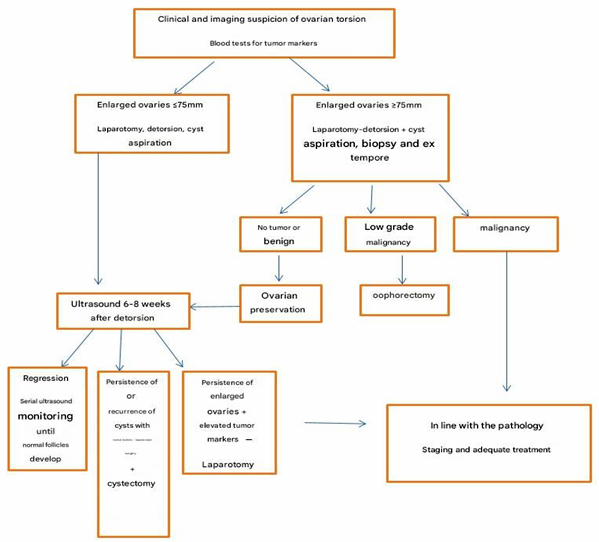
Treatment and assessment of ovarian viability
Treatment involves surgical management and at the same time
confirmation of the diagnosis. Early diagnosis and surgical therapy
are necessary in order to protect ovarian and tubal function and to
prevent more serious morbidity. Minimizing the total time during
which the ovary is in ischemia is a key component of therapy, but
the time required for ovarian necrosis to occur is unclear. [37,38].
Picture 1. Surgery of a torted ovarian fibroma
(Dr. Janković, General Hospital Pirot)
As long as the venous and lymphatic vessels are occluded, the
patient may have symptoms for some time before the arterial vessels
become occluded [30,40,41]. In a retrospective study of the
pediatric population, the median time to save the ovary before
detorsion was 10.8 hours. If detorsion is performed within the first
8 hours, the ovary is preserved in 40% of cases, and within the
first 24 hours in 33% [42]. This finding is consistent with data
showing that in women, the percentage of preserved ovaries is 30% if
surgery is performed within the first 24 hours from the onset of
symptoms [43,44]. Different studies show varying times from symptom
onset to detorsion in order to preserve the ovary. Animal studies
have shown that necrosis can occur 36 hours or more after occlusion.
Pediatric and adult populations show good long-term outcomes after
detorsion of either hemorrhagic or ischemic ovaries, with normal
follicle production later in life in 90–94% of cases described
[45,46]. There are two surgical treatment methods — laparoscopy and
laparotomy. Laparoscopy represents a reasonable alternative. The
benefits of laparoscopy include reduced need for analgesics, early
mobilization, cosmetic advantage, and earlier discharge to home
care. An additional advantage is that laparoscopic ovarian
cystectomy is associated with a lower incidence of postoperative
adhesions compared to laparotomy [47]. Laparotomy is recommended
when a malignant process is suspected. What is essential is the
assessment of ovarian viability and preservation of its function.
The only way to assess the viability of the ovary is by gross visual
inspection. Conventionally, a dark and enlarged ovary may be only in
venous or lymphatic congestion and may appear nonviable, but there
is a substantial probability that it is a viable ovary that can
regain function after detorsion [46]. There are other methods to
assess ovarian viability, such as injecting fluorescein and
observing the flow under ultraviolet light [48]. Another method is
ovarian bivalving, i.e., laparoscopically making an incision in the
ovary with an electric hook (L-hook) after detorsion and observing
whether there is blood flow at the cut surface. This also serves a
therapeutic purpose by reducing pressure caused by venous and
lymphatic congestion [49]. There is no precisely determined time for
ovarian necrosis to occur. A definitive sign of ovarian necrosis is
a gelatinous formation that disintegrates upon manipulation. What is
expected from surgical treatment? Ideally, detorsion [50], detorsion
with oophoropexy, ovarian cystectomy (recommended for benign cysts
after detorsion), or salpingo-oophorectomy in cases of suspected
malignancy, necrotic ovaries, and postmenopausal women.
Algorithm 2. Procedure in menopausal women
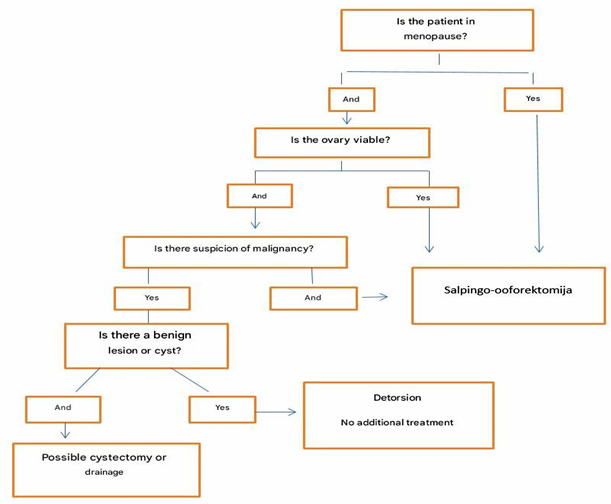
CONCLUSIONS:
- Ovarian torsion mainly affects women of reproductive age,
but a significant percentage also occurs in the premenarcheal
period, in pregnant women, and in postmenopausal women.
- Ovarian torsion occurs due to complete or partial rotation
of the ovary and fallopian tube, leading to obstruction of
vascular flow.
- Crucial factors for ovarian torsion include the presence of
an ovarian mass in women of reproductive age.
- Almost 90% of women experience abdominal pain that begins
suddenly, is sharp, and intermittent in nature.
- Up to 70% experience nausea and vomiting.
- Nearly one-third of patients with torsion have no abdominal
or pelvic pain on examination.
- Although ultrasound is used as the primary diagnostic
modality in the evaluation of ovarian torsion with high
specificity, a normal ultrasound finding cannot effectively rule
out the diagnosis.
- CT of the abdomen and pelvis with IV contrast can be helpful
in diagnosis.
- In the evaluation, findings for reduced or absent ovarian
enlargement, peripheral displacement of follicles, enlarged
ovaries with follicular stroma, and thickened fallopian tube are
considered significant
REFERENCE:
- Franco PN, García-Baizán A, Aymerich M, Maino C,
Frade-Santos S, et al. Gynaecological Causes of Acute Pelvic
Pain: Common and Not-So-Common Imaging Findings. Life
(Basel).2025;13(10):2025.
- Hibbard LT.Adnexal torsion. Am J Obstet Gynecol
1985;152:456-61.
- Bouguizane S, Bibi H, Farhart Y, et al. Adnexal torsion: a
report of 135 cases. J Gynecol Obstet Biol Reprod.
2003;32(6):535–40.
- Gibson E, Mahdy H. Anatomy, Abdomen and Pelvis, Ovary.
StatPearls Publishing, Treasure Island 2019.
- Ying J, Feng J, Hu J, Wang S, Han P, Huang Y, et al. Can
ovaries be preserved after an ovarian arteriovenous
disconnection? One case report and a review of surgical
treatment using Da Vinci robots for aggressive ovarian
fibromatosis. J Ovarian Res. 2019;12(1):52.
- Huang C, Hong MK, Ding DC. A review of ovary torsion. Tzu
Chi Med J. 2017;29(3):143-7.
- Sanfilippo JS, Rock JA. Surgery for benign disease of the
ovary. In: TeLinde's Operative Gynecology, 11th ed., Jones HW,
Rock JA (Eds), Wolters Kluwer, 2015.
- Beaunoyer M, Chapdelaine J, Bouchard S, Ouimet A.
Asynchronous bilateral ovarian torsion. J Pediatr Surg 2004;
39:746-9.
- Takeda A, Hayashi S, Teranishi Y, et al. Chronic adnexal
torsion: An under-recognized disease entity. Eur J Obstet
Gynecol Reprod Biol 2017; 210:45.
- Huchon C, Fauconnier A. Adnexal torsion: a literature
review. Eur J Obstet Gynecol Reprod Biol 2010; 150:8-12.
- Raicevic M, Saxena AK. Asynchronus bilateral ovarian
torsions in girls-systematic review. World J Pediatr 2017;
13:416-20.
- Oltmann SC, Fischer A, Barber R, et al. Cannot exclude
torsion – a 15-year review. J Pediatr Surg. 2009;44:1212–6.
- Smorgick N, Melcer Y, Sarig-Meth T, et al. High risk of
recurrent torsion in premenarchal girls with torsion of the
normal adnexa. Fertil Steril. 2016;105:1561–5.
- Breech LL, Hillard PJ. Adnexal torsion in pediatric and
adolescent girls. Curr Opin Obstet Gynecol. 2005;17:483–9.
- Heling KS, Chaoui R, Kirchmair F, et al. Fetal ovarian
cysts: prenatal diagnosis, management and postnatal outcome.
Ultrasound Obstet Gynecol 2002; 20:47-50.
- Ssi-Yan-Kai G, Rivain AL, Trichot C, et al. What every
radiologist should know about adnexal torsion. Emerg Radiol.
2018;25(1):51–9.
- Tsafrir Z, Hasson J, Levin I, et al. Adnexal torsion:
cystectomy and ovarian fixation are equally important in
preventing recurrence. Eur J Obstet Gynecol Reprod Biol 2012;
162:203-5.
- Houry D, Abbott JT. Ovarian torsion: a fifteen-year review.
Ann Emerg Med 2001; 38:156-9.
- White M, Stella J. Ovarian torsion: 10-year perspective.
Emerg Med Australas 2005;17:231-7.
- Sasso RA. Intermittent partial adnexal torsion after
electrosurgical tubal ligation. J Am Assoc Gynecol Laparosc
1996; 3:427-30.
- Huchon C, Fauconnier A. Adnexal torsion: a literature
review. Eur J Obstet GynecolReprod Biol. 2010;150(1):8–12.
- White M, Stella J. Ovarian torsion: 10-year perspective.
Emerg Med Australas. 2005;17(3):231–7.
- Sasaki KJ, Miller CE. Adnexal torsion: review of the
literature. J Minim InvasiveGynecol. 2014;21(2):196–202.
- Gasser CRB, Gehri M, Joseph JM, Pauchard JY. Is it ovarian
torsion? A systematic lit-erature review and evaluation of
prediction signs. Pediatr Emerg Care. 2016;32(4):256–61.
- P. Bolli, S. Schadelin, S. Holland-Cunz, P.
Zimmermann.Ovarian torsion in children:development of a
predictive score. Medicine 2017;96(43) :e8299.
- M. B. Pepys, G. M. Hirschfield. C-reactive protein: a
critical update. J ClinInvest 2003;111(12):1805-12.
- Cohen SB, Wattiez A, Stockheim D, et al. The accuracy of
serum interleukin-6 and tumour necrosis factor as markers for
ovarian torsion. Hum Reprod 2001; 16:2195-7.
- Daponte A, Pournaras S, Hadjichristodoulou C, et al. Novel
serum inflammatory markers in patients with adnexal mass who had
surgery for ovarian torsion. Fertil Steril 2006; 85:1469-72.
- Taufiq DM, Bharwani NM, Sudderuddin SA, Rockall AG, Stewart
VR. Adnexal torsion: review of radiologic appearances.
Radiographics. 2021;41(2): 609–24.
- Robertson JJ, Long B, Koyfman A. Myths in the evaluation and
Management of Ovarian Torsion. J Emerg Med. 2017;52(4):449–56.
- Bridwell RE, Koyfman A, Long B. High risk and low prevalence
diseases: Ovarian torsion. Am J Emerg Med. 2022 ;56:145-150.
- Moro F, Bolomini G, Sibal M, et al. Imaging in gynecological
disease (20): clinical and ultrasound characteristics of adnexal
torsion. Ultrasound Obstet Gynecol 2020; 56:934-43.
- Adnexal Torsion in Adolescents: ACOG Committee Opinion No,
783. Obstet Gynecol 2019; 134:e56. Reaffirmed 2021.
- Janković N, Janković M, Ristić Petrović A, Dimitrijević S. A
rare disease which lead to avoidable ovary loss. Ovarian
torsion-Case reports. Med. Pregl. 2025.(in press)
- Pignataro JN, Schindler L. Isolated Fallopian Tube Torsion:
Diagnosis and Management of a Gynecologic Emergency. Cureus.
2023;15(9):e46260.
- Dhanda S, Quek ST, Ting MY, et al. CT features in surgically
proven cases of ovarian torsion—a pictorial review. Br J Radiol.
2017; 90(1078):20170052
- Schlaff W, Lund K, McAleese K, Hurst B. Diagnosing ovarian
torsion with computed tomography. A case report. J Reprod Med.
1998;43(9):827–30.
- Spinelli C, Piscioneri J, Strambi S. Adnexal torsion in
adolescents: update and review of the literature. Curr Opin
Obstet Gynecol. 2015;27(5):320–5.
- Dasgupta R, Renaud E, Goldin AB, et al. Ovarian torsion in
pediatric and adolescent patients: A systematic review. J
Pediatr Surg. 2018;53(7):1387–91.
- Cass DL. Ovarian torsion. Semin Pediatr Surg.
2005;14(2):86–92.
- Cicchiello LA, Hamper UM, Scoutt LM. Ultrasound evaluation
of gynecologic causes of pelvic pain. Obstet Gynecol Clin North
Am. 2011;38(1):85–114.
- Anders JF, Powell EC. Urgency of evaluation and outcome of
acute ovarian torsion in pediatric patients. Arch Pediatr
Adolesc Med. 2005;159(6):532–5.
- Shalev J, Goldenberg M, Oelsner G, et al. Treatment of
twisted ischemic adnexa by simple detorsion. N Engl J Med.
1989;321(8):546.
- Oelsner G, Bider D, Goldenberg M, Admon D, Mashiach S.
Long-term follow-up of the twisted ischemic adnexa managed by
detorsion. Fertil Steril. 1993;60(6): 976–9.
- Cohen SB, Oelsner G, Seidman DS, Admon D, Mashiach S,
Goldenberg M. Laparoscopic detorsion allows sparing of the
twisted ischemic adnexa. J Am Assoc Gynecol Laparosc.
1999;6(2):139–43.
- Pansky M, Abargil A, Dreazen E, Golan A, Bukovsky I, Herman
A. Conservative management of adnexal torsion in premenarchal
girls. J Am Assoc Gynecol Laparosc. 2000;7(1):121–4
- Lundorff P, Hahlin M, Kallfelt B, Thorburn J, Lindblom
B.Adhesionformation after laparoscopy surgery in tubal
pregnancy: a randomizedtrial versus laparotomy. Fertil Steril.
1991;55 (5):911-5.
- McHutchinson LL, Koonings PP, Ballard CA, d'Ablaing G 3rd.
Preservation of ovarian tissue in adnexal torsion with
fluorescein. Am J Obstet Gynecol 1993; 168:1386-8.
- Styer AK, Laufer MR. Ovarian bivalving after detorsion.
Fertil Steril 2002; 77:1053-5.
- Wang JH, Wu DH, Jin H, Wu YZ. Predominant etiology of
adnexal torsion and ovarian outcome after detorsion in
premenarchal girls. Eur J Pediatr Surg 2010; 20:298-301.
|
|
|
|



