| |
|
|
INTRODUCTION Malignant mesothelioma is a relatively rare
but very aggressive tumor. It represents a multifactorial disease in
whose development the following factors play a role: asbestos,
Simian virus 40, and radiotherapy [1]. It has not been proven that
smoking causes the occurrence of MPM, but it contributes to its
development. According to data from the literature, its occurrence
is causally related to asbestos exposure as the leading etiological
factor that contributes to the development of the disease in more
than 80% of cases. It appears after inhalation of microscopic
asbestos mineral fibers suspended in the air, after a long latent
period of several decades. It has a much higher incidence in men,
which is explained by the fact that men are more often engaged in
occupations that are “risky” in terms of asbestos exposure.
Occupational exposure to asbestos has been the subject of numerous
studies. Such an association of MPM with occupation is most likely
the consequence of not implementing occupational safety measures. A
particularly concerning fact is the occurrence of mesothelioma in
family members of these workers. The disease also more frequently
appears in places where mines of this material are located, because
exploitation leads to contamination of the environment (air) and
exposure of the population to asbestos (endemic areas) [2]. As a
carcinogenic material, asbestos was banned in all European Union
member states in 2005, while Serbia introduced a ban on the use of
asbestos in all products in 2011, and a Regulation on handling waste
containing asbestos was also prescribed. [3].
CASE REPORT
The patient is a 64-year-old male. A retired machine locksmith.
Smoker for over 40 years, about 20 cigarettes per day, rarely
consumes alcohol. In his personal medical history previously
healthy, without other comorbidities. The patient states that he
felt the first symptoms at the beginning of June 2018. The main
complaints are shortness of breath, a feeling of choking, and
fatigue on minimal exertion. Cough has been present for several
months before that, since March 2018. Elevated blood pressure for
the past two weeks, BP 160/100 mmHg, previously normal blood
pressure. Evaluated by an internist, received antihypertensive
therapy and was further referred to a pneumophysiologist.
Auscultation of the lungs on the left reveals completely absent
breath sounds, without accompanying sounds, while in the other parts
of the lungs the breath sounds are normal. Blood oxygen saturation
is 97%.
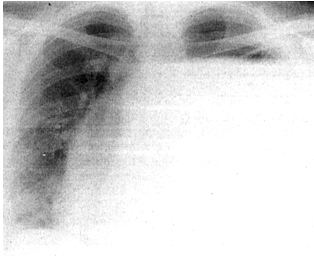
Figure 1. Initial chest radiograph
Chest radiography (Figure 1) indicates a left hydropneumothorax,
with an infraclavicularly present hydro-air level. The cardiac
silhouette is displaced to the right. All laboratory and biochemical
analyses are within reference values. After the basic laboratory and
diagnostic examinations performed at the Health Center Knjaževac,
the patient was further referred to the Special Hospital for
Pulmonary Diseases Ozren on 26.06.2018. Two days later he was
transferred to the Clinic for Thoracic Surgery, University Clinical
Center Niš, for further treatment, where he was hospitalized several
times in the following period. During the first hospitalization at
this clinic, an initial drainage of the left pleural space was
performed, with approximately 3000 ml of fluid evacuated.
Cytological and bacteriological analyses did not indicate the
presence of tumor cells or infection.
During the next hospitalization, surgery was performed on
24.07.2018., video-assisted thoracoscopy (VATS), partial pleural
decortication and biopsy were done, and the material was sent for
histopathological examination. In the histopathological report dated
11.09.2018., malignant pleural mesothelioma, epithelioid variant,
was diagnosed. On the follow-up PA chest radiograph from 16.09.2018.
(Figure 2), the left hemidiaphragm and left costophrenic angle are
obscured by a laterally ascending shadow—pleural effusion is
present. The remaining part of the lung parenchyma on the left shows
reduced transparency.
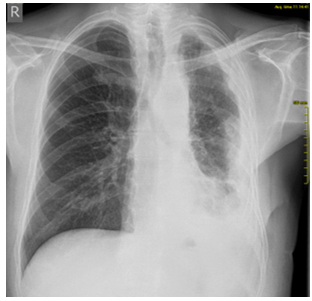
Figure 2. Control radiography of the chest
In the conclusion of the chest MSCT dated 24.09.2018: in the
pleural cavity on the left, a heterogeneous-density lesion is
present diffusely, with denser fluid content, accompanied by
compressive atelectasis and a soft-tissue component; the described
lesion primarily corresponds to a neoplastic process with empyema.
In the remaining lung parenchyma, micronodular changes and
mediastinal lymphadenopathy are present. In the bony structures,
apart from degenerative changes, there are no other MSCT findings.
According to the decision of the Pulmonology Oncology Council from
25.09.2018, treatment with first-line chemotherapy using the
pemetrexed–cisplatin regimen was planned. In the meantime, further
disease progression occurred. Poor appetite and progressive weight
loss were present. The appearance of central neurological symptoms
was suspected, including impaired communication, occasional
disorientation to time, instability, and intermittent loss of
sphincter control (occasional urinary incontinence). Pain was
constantly present. From analgesic therapy, Ibuprofen 600 mg tablets
2×1 and Tramadol 50 mg tablets 2–3×1 were administered, but due to
insufficient analgesic effect, the use of a Fentanyl transdermal
patch 25 micrograms/h was soon initiated. The patient was
hospitalized at the Oncology Clinic, University Clinical Center Niš,
on 15.10.2018. Laboratory and biochemical analyses showed azotemia
and hypercalcemia (urea 16.4 mmol/L, creatinine 179.4 μmol/L, Ca
4.26 mmol/L). Due to clinical deterioration and poorer performance
status, chemotherapy was not indicated. Because of the elevated
serum calcium levels, a decision was made to administer
bisphosphonates, and urgent treatment was initiated. Therapy with
Zoledronic acid 4 mg ampoule was administered without complications.
However, progressive central neurological deterioration ensued, and
the patient died before the first cycle of the planned systemic
therapy.
Due to the specific nature and diversity of his occupations,
asbestos exposure was likely present on multiple occasions
throughout his life. However, the most probable critical exposure to
asbestos may have occurred 30 to 35 years earlier.
DISCUSSION
Malignant mesothelioma can have different localizations and
arises from mesothelial cells of serous membranes that line body
cavities and organs (visceral or parietal pleura, peritoneum,
pericardium, or, rarely, the coverings of other organs, e.g., the
tunica vaginalis of the testis). Most commonly diagnosed is
malignant pleural mesothelioma (MPM), accounting for over 70% of
cases. The tumor appears after a very long latent period of several
decades. The time from asbestos exposure to tumor development is at
least 25 years, and according to some authors, more than 50 years.
Due to this long latent period, MPM is most often diagnosed in
patients over 60 years of age. Among patients with confirmed
high-risk occupations, the most common were machine-fitters, as in
our patient [1,4].
Asbestos includes six naturally occurring silicate minerals. It
consists of soft, thin, silky fibrous crystals. There are two types
of asbestos fibers: amphibole (most commonly used: crocidolite or
blue; amosite or brown asbestos; fibers are long, thin, and straight
– needle-like) and serpentine (chrysotile or white asbestos; fibers
have a serpentine shape) [5]. Asbestos was widely used worldwide,
especially in the second half of the last century. Due to its
favorable physical properties, it had broad applications: it is a
good conductor of heat, does not burn or carbonize, and is durable.
All forms of asbestos fibers can be responsible for disease
development. Some forms are more pathogenic than others. Thinner and
longer fibers have the greatest carcinogenic potential. All types of
asbestos are very stable and do not degrade spontaneously over time;
however, processing or damage produces asbestos dust. This dust is
easily inhaled and reaches the alveolar sacs. The exact mechanism by
which asbestos fibers reach the pleura and mesothelial cells is not
fully clarified. Over a long period, these fibers cause chronic
inflammation, fibrosis, and malignant alterations. Oncogenesis is
not fully understood. MPM most likely arises as a result of
inactivation of tumor suppressor genes. The most frequent changes
are loss of function of the CDKN2A tumor suppressor gene, NF2
inactivation, and mutation or deletion of the BAP-1 tumor suppressor
gene (BRCA1-associated protein 1) [6]. All these factors together
contribute to the development of MPM.
Symptoms of mesothelioma vary depending on the localization and
stage of the disease. After a long latent period, initial symptoms
are usually nonspecific and mild. In pleural mesothelioma,
complaints include breathing difficulties, progressive dyspnea, and
rapid fatigue with minimal exertion. In our patient, all these
symptoms were present. The cough was dry and exhausting, and
hemoptysis (coughing up blood) may occur. Our patient did not have
hemoptysis, but the cough was present. Some symptoms may result from
pleural effusion. Elevated blood pressure is not described as a
symptom of MPM, but in our patient, it was likely a consequence of
massive left-sided pleural effusion. Pleural effusions can be
massive and often recurrent. During effusion drainage, transient
relief occurs, followed by pain. Chest pain is the leading symptom
of MPM and is thought to result from tumor infiltration into
surrounding structures. Severe chest pain was also present in our
patient. Neurological symptoms in the patient may be a possible
consequence of disease dissemination to the CNS.
Some general symptoms, such as malaise, general weakness, fatigue,
loss of appetite, and weight loss, were also present in our patient
during the later course of the disease. Occasionally, fever, chills,
and night sweats may occur, usually in advanced stages of the
disease.
Standard chest radiography is a first-line diagnostic method,
although it is not sufficiently sensitive or specific. A common
finding on radiography is the presence of a unilateral pleural
effusion. Cytological analysis of punctured pleural fluid has a
sensitivity ranging from 13% to 75%, but it may be negative or
false-negative. In the described patient, it was negative. Chest CT
is an indispensable diagnostic procedure that provides valuable
information about the pleura (thickening, calcifications),
characteristics of effusions (if present), and the condition of
mediastinal lymph nodes and organs.
Percutaneous biopsy is both a diagnostic and therapeutic procedure
for MPM. Video-assisted thoracoscopy (VATS) is the most reliable
method, providing an adequate sample for morphological and
immunohistochemical analysis. Macroscopically, these tumors appear
as diffuse pleural thickening. Pathohistological diagnosis is the
gold standard. Histologically, tumors are divided into subtypes:
epithelioid, sarcomatoid, and biphasic, which consists of a mixture
of the two types [1,4]. In our patient, the diagnosis of epithelioid
MPM was established, which is the most frequent type, accounting for
about 60% of cases. It has a better prognosis, responds better to
therapy, and has longer average survival. Although there are three
histological subtypes of MPM, the WHO in 2021 proposed a complex and
comprehensive classification of pleural and pericardial tumors
[7,8], taking into account histological characteristics, prognosis,
disease extent, BAP1 tumor suppressor gene immunohistochemistry,
CDKN2A homozygous deletion, and other factors.
The therapeutic approach is based on a multimodal strategy,
combining surgery, chemotherapy, radiotherapy, and immunotherapy.
Despite significant progress in recent years, treatment options
remain limited. Current therapeutic modalities prolong survival but
do not provide complete cure.
Operability depends on tumor size and the patient’s general
condition. Stages I to IIIa are operable if the tumor is still
localized and of the epithelioid type. In later stages with
metastases, surgery has a palliative benefit.
Chemotherapy is the most commonly used treatment modality for
mesothelioma and is applied in all stages. Pemetrexed and cisplatin
constitute the first-line chemotherapy regimen. Some patients may
respond better to other recommended combinations, such as pemetrexed
with carboplatin, or cisplatin with gemcitabine [9]. Radiotherapy
has most often been applied palliatively to relieve symptoms in
later stages of disease, although technical advances have allowed
significant improvements in MPM management [10]
In recent years, immunotherapy with monoclonal antibodies has been
implemented. Nivolumab in combination with ipilimumab is indicated
as first-line treatment for patients with unresectable MPM.
Treatment continues until disease progression, unacceptable
toxicity, or for up to 24 months. Patients treated in this way have
shown significant improvement [11].
Malignant pleural mesothelioma is a highly aggressive tumor. The
disease is incurable in later stages, and the prognosis is always
very poor. Expected survival is less than 18 months from the onset
of initial symptoms, while survival for advanced disease without
therapy is 6–8 months.
According to a study conducted in 2019 in an endemic area of Turkey
[2], postoperative survival results showed a median survival period
of 19.6 months. Among 13 patients, the longest survival of 32 months
was observed in a patient who underwent postoperative hyperthermic
chemotherapy after pleural decortication.
CONCLUSION
There are anamnesis data that deserve attention: sex, age (over
60 years, due to the long latent period), occupation, and smoking
history. The described case represents a typical patient with MPM,
who initially presented with typical nonspecific symptoms such as
cough, dyspnea, and fatigue, along with a characteristic unilateral
pleural effusion, and later developed severe chest pain and rapid
disease progression. The disease is most often diagnosed at an
advanced stage. Anamnestic data regarding possible asbestos exposure
during life may raise suspicion and contribute to an earlier
diagnosis, at a stage when therapeutic options are somewhat greater
and may, for a limited time, prolong survival. The most important
measure is primary prevention: to prevent asbestos exposure or to
safely remove asbestos-containing material. MPM is a rare disease,
but it should be considered in the differential diagnosis, as it is
a highly aggressive tumour.
LITERATURE:
- Uroš P. et al. Kliničko–patološke karakteristike malignog
pleuralnog mezotelioma - Naše petogodišnje iskustvo. MD-Medical
Data. 2019;11(1):019-22.
- Kermenli Tayfun, Azar Cebrail. Rezultati postoperativnog
preživljavanja pacijenata sa pleuralnim malignim mezoteliomom
stadijuma I-II u endemskom području. Sanamed. 2020;15(2):139-44.
- Pravilnik o otpadu koji sadrži azbest Sl.glasnik RS, br
75/2010
https://www.ekologija.gov.rs/sites/default/files/old-documents/Otpad/Pravilnici/PRAVILNIKopostupanjusaotpadomkojisadazbest.pdf
[homepage on the Internet]
- Martina M et al. Novosti u dijagnostici malignog mezotelioma
pleure. Medicina fluminensis. 2021;57(3):254-59.
- Kanceljak-Macan B. Imunological aspects of asbestos-related
diseases Arh Hig Rada Toksikol 2009;60:45-50.
- Anna-Mariya Kukuyan et al. Inactivation of Bap1 Cooperates
with Losses od Nf2 and Cdkn2a to Drive the Development of
Pleural malignant Mesothelioma in Conditional Mouse Models
PubMedCentral 2019 May 31; Author Manuscript
2019;79(16):4113-4123.
- Jennifer S, Sanja D, Francoise G-S, Richard A, Kelly B,
Andrew H.The 2021 WHO Classification of Tumors of the Pleura:
Advances Since the 2015 Classification. Jurnal of Thoracic
Onkology. 2022;17(5):608-22.
- Valerie R, Kari C, Hedy K, Anna N, Harvey P, David R et al.
The IASLC Mesothelioma Staging Project: Proposals for the M
Descriptors and for Revision of the TNM Stage Groupings in the
Forthcoming (Eighth) Edition of the TNM Classification for
Mesothelioma. Jurnal of Thoracic Onkology. 2016;11(12):2112-19.
- David s. Ettinger et al. Malignant Pleural Mesothelioma PMC
2023 May 16; Author Manuscript 2016;14(7):825-836.
- Tatjana A, Aleksandar S, Marina N. Uloga radioterapije u
lečenju malignog mezotelioma pleure-mogućnosti i kontroverze.
Srpski arhiv za celokupno lekarstvo.Časopis srpskog lekarskog
društva 2024;152(1-2):92-96.
-
https://www.alims.gov.rs/doc_file/lekovi/smpc/515-01-00472-21-002.pdf
[homepage on the Internet]
- Michele C, Shreya K, Ann C, Aubrey M, Anil W, David W et al.
Consensus Report of the 2015 Weinman International Conference on
Mesothelioma. Journal of Thoracic Oncology. 2016;11(8):1246-62.
- https://www.asbestos.com/[homepage on the Internet]
- Mirjana A, Jovica J.Medicina rada, Univerzitet u Nišu-
Medicinski fakultet, 2009.
- Paul B, David R. IASLC Thoracic Oncology. 2nd ed; 2018.
- Gary A. Ulaner. Pleura on FDG PET/CT. Fundamentals of
Oncologic PET/CT. 2019.
- The Surveillance, Epidemiology, and End Results (SEER)
Program Mesothelioma - Cancer Statistics Review. Available from:
https://seer.cancer.gov/csr/1975_2016/results_merged/sect_17_mesothelioma.pdf
- Journal of Thoracic Oncology, 2016 Malignant Pleural
Mesothelioma In Specialty Imaging: Thoracic Neoplasms, 2016.
|
|
|
|


