| |
|
|
INTRODUCTION
Acute bacterial meningitis (ABM) is an infectious disease with
significant morbidity and mortality worldwide. Mortality in
untreated patients is up to 50%, in treated 8-15%. Having gone
through the disease, 10-20% of patients remain with permanent
neurological and mental disorders. [1]. Etiological agents depend on
age and geographical area. Streptococcus pneumoniae and Neisseria
meningitidis are the most common causes of ABM in adults [2].
Hemophilus influenzae is the cause of ABM at all ages, more common
in the population of children up to 5 years of age before the
mandatory vaccine [3]. The etiological diagnosis requries isolation
of the causative agent from the cerebrospinal fluid (CSF), but
meningism is possible with the presence of bacteria in the blood
[4]. Predisposing factors for the development of ABM include head
trauma, sinusitis, otitis, pharyngitis, pneumonia, but also other
immunodeficient conditions such as alcoholism, splenectomy,
neurological and hematological diseases.
THE AIM of this study was to analyze the epidemiological
characteristics, etiology, risk factors, clinical course and
prognosis of acute bacterial meningitis in the adult population in
the Zlatibor district.
MATERIAL AND METHODS
The research included patients treated at the Department of
Infectious Diseases and the Intensive Care Unit of the General
Hospital Uzice, in the period from 1st. January 2009 to 31st
December 2019. Demographic data, risk factors, hematological and
biochemical data from blood and CSF, cytological findings of CSF
were collected retrospectively. The clinical course and outcome of
the disease were analyzed, too.
Hematological and biochemical analyses from blood and CSF were
performed by standard methods used in the Republic of Serbia. The
etiological diagnosis was made by identifying the causative agent
from CSF culture or blood, when CSF culture was negative or
unavailable. Samples of CSF were cultured on blood agar plates
containing 5% sheep blood and on chocolate agar, incubated in carbon
dioxide for 24 - 48 h at 37° C. Isolates of Streptococcus pneumoniae
and Neisseriae meningitidis were preliminarily identified based on
typical colonial prospects, Gram staining and optochin test for
Streptococcus pneumoniae. The Vitek system (bioMérieux, Marcy
l'Etoile, France) was used for the final identification and testing
of antibiotic susceptibility. The minimum inhibitory concentration
test was performed by the E test, according to CLSI guidelines [5].
All patients underwent ophthalmic examination of the fundus and/or
computed tomography (CT) scan of the endocranium.
Patients with tuberculous meningitis were excluded from the study.
The outcome of the disease was assessed on the basis of the Glasgow
Coma Scale with the following values:
score 1 - death; score 2 - inability of patients to interact with
the environment; score 3 - inability of the patient to live
independently, but there is an interaction with the environment;
score 4 - ability to live independently with incapacity for work;
score 5 - working ability. The favorable outcome of the disease was
defined by a score of 5, while scores 1 to 4 were marked as an
unfavorable outcome [6].
The Statistical Package for Social Sciences SPSS (version 16.0) was
used for statistical analysis. A significant difference was
represented by P <0.05.
RESULTS
A total of 148 patients with ABM was examined, 92 men and 56
women, aged 22 to 84 years of age, averaged 55.8 +/- 13.1.
A significant number of patients had comorbidities. A third of
patients had diabetes and heart disease, 22.3% consumed alcohol
excessively. The origin of the infection could be assumed in 88.5%
of the patients. Sinusitis was significantly the most common at
41.9%. In 19.6% of patients, ABM was preceded by ear inflammation,
in 12.8% by pharyngitis. All patients experienced headache on
admission, 97.2% had fever, and 95.9% had neck stiffness during head
anteflexion. Vomiting and photophobia were present in 76.3% and
75.6%, respectively. There was no statistically significant
difference between the presence of these symptoms.
All patients underwent ophthalmologic examination of the fundus. CT
scan of the endocranium was performed in 82.4%. Pathological finding
in the sinus cavities was significantly the most common in 41.9%.
Epidemiological characteristics, comorbidities, possible focus of
infection, symptoms and findings of CT scan of the endocranium are
shown in Table 1.
Table 1. Epidemiological parameters, comorbidities,
focus of infection, symptoms and CT scan finding in patients with
ABM
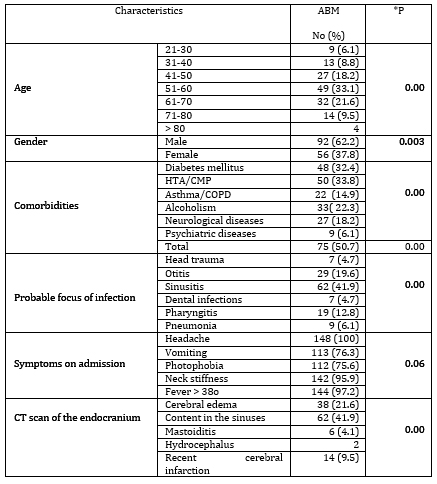
*P - statistical significance for samples ≥ 5
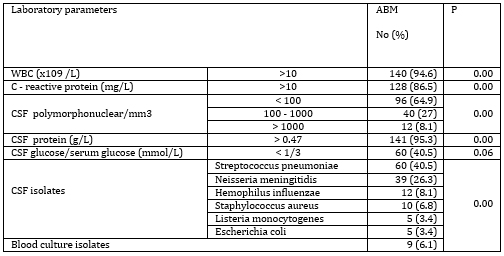
All patients underwent lumbar puncture. The number of
polymorphonuclear leukocytes was significantly up to 100/mm3. In a
significant majority of patients (95.3%), CSF proteins were
elevated, while CSF/blood glucose index was reduced in 40.5% of
subjects. The value of protein in the CSF was from 0.22 - 6.1 g / L,
on average 2.8 +/- 2.2 g / L.
The most common causes of ABM were Streptococcus pneumoniae and
Neisseria meningitidis, in 40.5% and 26.3%, respectively. Other
pathogens were significantly rarer.
Serum biochemical parameters of bacterial infection, leukocytosis
and elevated C-reactive protein (CRP) level, were observed in a
significant number of patients, 94.6% and 86.5%, respectively. The
leukocyte count ranged from 5.6 to 16.2x109 / L, averaging
12.4x109/L. The CRP value range was 3.4 - 122 mg/L, averaging 34.1
+/- 45.2 mg / L.
Biochemical findings from blood and cerebrospinal fluid, cytological
findings of cerebrospinal fluid and etiological causes of acute
bacterial meningitis are shown in Table 2.
Table 2. Biochemical findings of blood and CSF,
cytological findings of CSF and etiological agents of ABM
The clinical course was favorable in a significant
majority (74.3%) of the patients. One third of patients had
epileptic seizures. In 24 (16.2%) patients the disease ended
lethally (Table 3).
Table 3. Clinical course and outcome of patients
with ABM
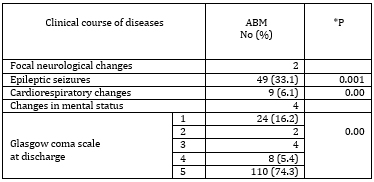
*P - statistical significance for samples ≥ 5
Risk factors for unfavorable disease outcome were further
examined (Table 4)
Table 4. Risk factors for unfavorable outcome of
ABM
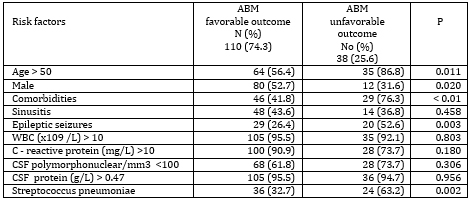
Significant factors for the unfavorable outcome of acute
bacterial meningitis were the presence of comorbidities,
Streptococcus pneumoniae as the cause of the disease, the occurrence
of epileptic seizures, age over 50 and male gender.
DISCUSSION
Analysis of the causes of ABM has indicated differences depending
on a wide range of examined age groups in recent years [7].The most
common causes are Streptococcus pneumoniae and Neisseria meningitis
in adult population, while in children the most common are
Streptococcus agalactiae, Escherichia coli, Listeria monocytogenes
[8]. Our study included adult population and the frequency of
individual pathogens corresponds to the above conclusion of other
researchers. Haemophilus influenzae is a childhood pathogen,
significantly rarer after the vaccine became mandatory [8]. In our
study, it was present in 8.1%, which is expected given that it is
the most common colonizer of the respiratory tract mucosa, and
especially common in persons with chronic obstructive pulmonary
disease [9].
Demographic data showed that men got sick more often, which
corresponds to the finding of Diaz and colleagues who proved that
men with ABM have more frequent head trauma and excessive alcohol
consumption as risk factors [10]. The main risk factor of our
patients was hypertension/cardiomyopathy. This can be explained by
older age of the patients. Diabetes mellitus was the second most
important risk factor. Diabetes leads to changes in body's immune
defenses. The function of polymorphonuclear leukocytes is reduced,
especially when acidosis is also present. Leukocyte adhesion,
chemotaxis, and phagocytosis were also altered and antioxidant
bactericidal systems were weakened [11].
Inflammation of the sinuses and ear are more common possible sources
of infection in our patients, unlike other studies [12]. This result
can be explained by the proven high percentage of bacterial
sinusitis in the adult population [13, 14]. This finding is
supported by the finding of CT scan, which most often indicated a
pathological process in the sinuses. In addition to headache, neck
stiffness and fever with a change in mental status were the most
common symptoms of both our study and others. [12]. The clinical
course of our patients was accompanied by the occurrence of
epileptic seizures in one third of patients. CNS infections as a
cause of epilepsy are present in a quarter of patients with ABM
[15]. Epileptic seizures have been shown to correlate with lower
sugar values and higher protein values in CSF [16]. Risk factors for
subsequent unprovoked seizures include focal discharge, sharp
electroencephalographic waves, and initial CSF glucose <20 mg / dl
[17]. The cytological finding of CSF with pleocytosis with the
dominance of polynuclear neutrophils is a standard finding in ABM,
which corresponds to our results. Elevated protein values present in
a significant majority of our patients are expected findings for
bacterial meningitis, although there are data in literature that
1-10% of patients with ABM do not have elevated CSF protein [18].
The value of glucose in CSF was reduced in 40% of our patients. This
is consistent with other data describing less than 50% of patients
with similar findings. The results indicate the unreliability of
this parameter in the diagnosis of bacterial meningitis [19].
The serum parameter of inflammation, C - reactive protein, was
elevated in a large percentage of our patients. Browver and
coauthors pointed out the unreliability of CRP values in the
diagnosis of ABM [20].
The unfavorable clinical course in our patients is smaller than
described [12]. The most common cause of death is Streptococcus
pneumoniae. The most significant risk factors are advanced age and
the presence of comorbidities, which corresponds to the findings of
other authors [12]. Our finding is partly consistent with the
findings of other authors who mention the most common age over 65
years [12]. Respondents from Nis authors were mostly of the same age
group as ours, with researchers noting that older people often have
more sparse symptoms at the beginning of the disease [21]. The
authors explain the small percentage of bacterial isolates from CSTs
by using antibiotic therapy before taking CSTs. This can delay the
diagnosis and adversely affect the further clinical course and
outcome of the disease. Interesting are the conclusions of the
authors who examined the influence of climatic factors on the
occurrence of bacterial meningitis and obtained a positive
correlation with the occurrence of wind and fog, and a negative
correlation with insolation [22]. It can be assumed that it would be
useful to analyze climate data in our patients as well.
CONCLUSION
The expected causative agent of a disease in a patient population
is of great importance for each geographical area. The most common
cause of acute bacterial meningitis in the adult population of
Zlatibor district is Streptococcus pneumoniae, in 40.5% of patients,
which is also the most common cause of adverse disease outcomes. The
second most common is Neisseria meningitidis (26.3%). ABM is most
common in men in their sixth decade of life who have comorbidities.
The occurrence of epileptic seizures during ABM is also a risk
factor of unfavorable outcomes of disease. The sourse of ABM is most
often in the sinuses or ear, so timely treatment of these infections
is an important preventive measure. Since there is a vaccine
prophylaxis for Streptococcus pneumoniae and Neisseria meningitidis,
it is necessary to recommend this preventive measure to the elderly,
especially those who have comorbidities.
REFERENCES
- World Health Organization (WHO). Meningococcal meningitis:
Fact sheet 2017 [updated December 2017; cited 2017 November 9].
Dostupno na:
http://www.who.int/mediacentre/factsheets/fs141/en/
- Centers for Disease Control and Prevention (CDC). Bacterial
Meningitis 2017 [updated January 25, 2017]. Dostupno na:
https://www.cdc.gov/meningitis/bacterial.html
- World Health Organization (WHO). Haemophilus influenzae type
b (Hib) Vaccination Position Paper July 2013. Releve
epidemiologique hebdomadaire. 2013; 88 (Suppl 39): 413-26.
- McGill F, Heyderman RS, Michael BD, et al. The UK joint
specialist societies guideline on the diagnosis and management
of acute meningitis and meningococcal sepsis in immunocompetent
adults. J Infect. 2016; 72: 405–38.
- European Committee on Antimicrobial Susceptibility Testing,
Breakpoint tables for interpretation of MICs and zone diameters,
2011 EUCAST Version 1.3. Available from:
http://www.eucast.org/clinical_breakpoints/
- Jennett B, Teasdale G. Management of head injuries. 2nd ed.
Philadelphia: F.A. Davis, 1981.
- Oordt-Speets AM, Bolijn R, van Hoorn RC, Bhavsar A, Kyaw MH.
Global etiology of bacterial meningitis: A systematic review and
meta-analysis. PLoS ONE 2018;13(6):e0198772. Available from:
https://doi.org/10.1371/journal.pone.0198772
- Brouwer MC, Tunkel AR, van de Beek D. Epidemiology,
diagnosis, and antimicrobial treatment of acute bacterial
meningitis. Clinical microbiology reviews. 2010; 23 (Suppl 3):
467-92.
- Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ.
Haemophilus haemolyticus: a human respiratory tract commensal to
be distinguished from Haemophilus influenzae. J Infect Dis.
2007; 195 (Suppl 1): 81-9.
- Yerramilli A, Mangapati P, Prabhakar S, Sirimulla H,
Shravani Vanam S, Voora Y. A study on the clinical outcomes and
management of meningitis at a tertiary care centre. Neurol
India. 2017; 65 (Suppl 5):1006-12.
- Joshi N, Gregory M. Caputo, Michael R. Weitekamp, A.W.
Karchmer. Infections in patients with diabetes mellitus.N Eng J
Med. 1999; 341: 1906-12.
- Beek D, Gans J, Spanjaard L, Weisfelt M, Reitsma JB,
Vermeulen M. Clinical Features and Prognostic Factors in Adults
with Bacterial Meningitis. N Engl J Med. 2004; 351: 1849-59.
- Sami AS, Scadding GK, Howarth P. A UK Community-Based Survey
on the Prevalence of Rhinosinusitis. Clin Otolaryngol. 2018;43 (Suppl
1): 76-89.
- Bhattacharyya N, Gilani S. Prevalence of Potential Adult
Chronic Rhinosinusitis Symptoms in the United States.
Otolaryngol Head Neck Surg.2018; 159 (Suppl 3): 522-5.
- Preux PM, Druet-Cabanac M. Epidemiology and etiology of
epilepsy in sub-Saharan Africa. Lancet Neurol 2005; 4: 21-31.
- Chang CJ, Chang HW, Chang WN, Huang LT, Huang SC, Chang YC,
Hung PL, Chang CS, Chuang YC, Huang CR, Tsai NW, Tsui HW, Wang
KW, Lu CH. Seizures complicating infantile and child-hood
bacterial meningitis. Pediatr Neurol 2004; 31: 165-71.
- Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and
other neurologic sequelae of bacterial meningitis in children.
NEngJ Med. 1990; 323: 1651-7.
- Viallon A, Botelho-Nevers E, Zeni F. Clinical decision rules
for acute bacterial meningitis: current insights. Open Access
Emergency Medicine 2016; 8: 7-16.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial
menin¬gitis in adults. A review of 493 episodes. N Engl J Med.
1993; 328 (Suppl 1): 21-8.
- Brouwer MC, Thwaites GE, Tunkel AR, Van De Beek D. Dilemmas
in the diagnosis of acute community-acquired bacterial
meningitis. Lancet. 2012; 380 (9854): 1684-92.
- Ranković A, Vrbić M, Jovanović M, Popović-Dragonjić L,
Đorđević-Spasić M. Meningeal syndrome in the practice of
Infectious diseases. Acta Medica Medianae 2017; 56(2): 32-7.
- Janković Lj, Pantović V, Damjanov V. Korelacija između klime
i bakterijskog meningitisa. Medicus 2006;7 (1):29-31.
|
|
|
|




