| |
|
|
INTRODUCTION
Assisted reproduction technologies are technologies that are
applied today, in the treatment of infertility, on human germ cells
and embryos. Currently in the Republic of Serbia, there are various
procedures of assisted reproduction technologies that are used to
treat infertility in patients depending on medical indications. The
availability of assisted reproduction technologies has evolved over
the years, and their application differs in biomedically assisted
fertilization centers that are in the Network Plan (state
institutions) and outside the Network Plan (private institutions).
Assisted reproductive technology (ART) is a group of
state-of-the-art therapeutic procedures for the treatment of
infertility [1].
Assisted reproductive technologies
ART refers to all technologies used to manipulate gametes outside of
the human body. Those most commonly used are in vitro fertilization
(IVF) and intracytoplasmic sperm injection (ICSI). They do not
include technologies such as intrauterine insemination (IUI) that
manipulates only male gametes [2]. Innovative technologies that
increase the success rate have also been developed. Some of them are
in vitro maturation (IVM), preimplantation genetic diagnosis (PGD),
sperm and oocyte donation (SD, OD) [3], frozen embryo transfer (FET)
procedure and assisted hatching (AH).
Medical indications for different assisted reproductive technologies
In vitro fertilization (IVF) is an assisted reproductive technology
that may be applied only to patients whose spermogramme shows
normospermia. Cultivated sample is then laid among the egg cells and
fertilization occurs alone. Medical indications for IVF require for
the male patient’s sample to show normospermia [4], and indications
for the female patient require blocked Fallopian tubes, ovulation
problems, endometriosis [5] and genetic diseases that result in
miscarriage [2].
Intracytoplasmic sperm injection –ICSI is a technology of
micromanipulation where one sperm is injected in the egg cell
cytoplasm, thus fertilizing it. Medical indications for ICSI are
mostly connected to male infertility, as well as patients who
haven’t achieved fertilization through IVF [5]. Male infertility
comprises oligospermia, asthenospermia, teratospermia, obstructive
and non-obstructive azoospermia, when sperms are collected
surgically (PESA – percutaneous epididymal sperm aspiration, TESA /
TESE – testicular sperm aspiration / extraction ). In the case of
the presence of antispermatozoal antibodies in both partners, the
ICSI method is performed, and after thawing frozen seed samples, the
microfertilization method is also applied [2].
Frozen embryo transfer (FET) is an embryo transfer obtained in one
of the previous procedures by the classical IVF or ICSI method,
followed by frozen vitrification processes. In the FET process, the
embryos are thawed and returned to the previously prepared
substance. Advantages of FET procedure lie in the fact that the
excess embryos from IVF procedures is frozen and then transferred in
successive cycles, which enables high cumulative rate of in vitro
fertilization [6].
In vitro maturation – IVM is a cycle in which egg cells are gathered
from antral follicles of unstimulated or mildly stimulated ovaries.
Immature ova are gathered, and the last phase of their maturation is
done under laboratory conditions. Medical indications for IVM cycles
include patients with polycystic ovary syndromes (PCOS) so as to
decrease the risk of ovarian hyperstimulation [7]. In patients with
estrogen-dependent cancers (oncology patients), stimulated cycles
with standard ovulation stimulation protocols are avoided because
they stimulate follicle growth and stimulate estrogen production, so
eggs are collected from antral follicles of unstimulated ovaries.
Preimplantation genetic diagnosis – PGD is a micromanipulation
technology done by biopsying several embryo cells 5-6 days old,
followed by analyzing genetic material of biopsied cells. Medical
indication for PGD includes patients with high risk of passing down
hereditary diseases to child, patients with repeated miscarriages
and patients above 38 years of age with risk of aneuploidy.
Assisted haching – AH is a micromanipulation technology by which
zona pellucidia on embryo is pierced so as to facilitate its
release, which increases implantation, as well as pregnancy rates.
Medical indications include multiple failed in vitro procedures, as
well as multiple failed transfers of frozen embryos.
Frozen oocyte replacement – FORs [8] are cycles which use frozen ova
(Oocyte cryopreservation – OoC) [9].
Oocyte donation (OD) represents inseminating ova of the female donor
with sperms taken from a male partner. The child’s genetics comes
from the male partner. Medical indications for oocyte donation
include premature ovarian failure (POF), poor quality of ova and
oncologically treated patients.
Sperm donation (SD) represents inseminatinf ova from a female
partner with sperms coming from a donor. Medical indications for
sperm donation are azoospermia, or other sperm abnormalities.
ART financing
Public financing among countries is available for an entire series
of reproductive technologies, including IVM, PGD, AH, OD, SD. Seven
countries (Denmark, France, Slovenia, Sweden and the UK (England,
Scotland, Wales) fullyor partially finance IVM trhough national
health programs. Twenty-two countries (Australia, Austria, Belgium,
Bulgaria, the Czech Republic, Denmark, Finland, France, Greece,
Hungary, Israel, Italy, Latvia, New Zealand, Norway, Russia, Spain,
Sweden, the UK (England, Scotland, Wales) fully or partially finance
PGD through their national health programs. There is no documented
evidence that the AH expenses are paid through public financing, and
there is no documented evidence that the expenses sperm or ovum
donation for in vitro are paid through national financing program
[3].
ART financed by the National health insurance fund is done in
Fertility centers from the Network plan and there are no other
options for technologies that cannot be invoiced through National
health insurance fund’s forms [10]. Technologies such as IVF and
ICSI were funded until 2017, and ever since then the National health
insurance fund has financed new technologies, such as FER procedure,
as well [11,12]. Fertility centers outside the Network plan offer
some other mentioned ART technologies apart from IVF and ICSI that
are financed by patients themselves.
According to Article 23 of the Law on the Treatment of Infertility
Procedures of Biomedical Assisted Fertilization, (“Official Gazette
of the Republic of Serbia”, No. 40/2017 and 113/2017, etc.), a
Fertility Center must keep medical records sent to the biomedicine
Board. Those records delivered to the Board for biomedicine include
data on all ART technologies. Those forms are delivered to the Board
for biomedicine, stating which technologies have been used in ART
procedures and this is recorded in the state register.
The aim of this article is to analyze available ART methods in
Fertility Centers within and outside the Network plan regulated by
the Law on the Treatment of Infertility Procedures of Biomedical
Assisted Fertilization (Official Gazette of the Republic of Serbia”,
No. 72/2009), their financing and availability to patients in the
Republic of Serbia.
MATERIAL AND METHODS
This article is assembled upon seeking documents using the
Internet and based on analyzed literature available on the Internet.
The results were gathered by analyzing official ART centers’
websites and analyzing available external secondary data from the
National health insurance fund and the Institute for public health
“Dr Milan Jovanovic Batut”.
RESULTS
Fetility Centers with whom the National health insurance fund has
concluded the contract on providing infertility treatments are:
• Fertility Centers within the Network plan:
- Clinic for Gynecology and Obstetrics, Clinical Centre of
Serbia, Belgrade
- Gynecology and Obstetrics Clinic, Clinical Centre of
Vojvodina, Novi Sad
- Obstetrics and Gynecology Clinic, Clinical Centre of Nis,
Nis
- Obstetrics and Gynecology Clinic “Narodni Front”, Belgrade
- Gynecology and Obstetrics Center, General Hospital of
Valjevo, Valjevo
- Clinic of Gynecology and Obstetrics, Clinical Center of
Kragujevac, Kragujevac
• Fertility Centers outside the Network plan:
- Special Gynecological Hospital for Treatment of Infertility
“Nikolov”, Kragujevac
- Special Hospital for Infertility Treatment “Spebo Medical”,
Leskovac
- Speical Hospital for Gynecology “Perinatal“, Novi Sad
- “Ferona” IVF Clinic, Novi Sad
- Special Hospital for Gynecology “GINS”, Novi Sad
- Special Gynecological Hospital “Genesis“, Novi Sad
- Special Gynecological Hospital “Teofanović“, Belgrade
- Special Gynecological Hospital “Beograd“, Belgrade
- Special Gynecology Hospital with Maternity Ward “Jevremova“,
Belgrade
- General Hospital “Analife“, Belgrade
- Special Hospital for Infertility Treatment “Intermedicus Bis“,
Belgrade
Fertility Centers in the Republic of Serbia have access to all
the important technologies for ART. ART technologies available in
Fertility Centers within and outside the Network plan are displayed
on Chart 1.
Chart 1. ART technologies available in Fertility
Centers within and outside the Network plan in the Republic of
Serbia
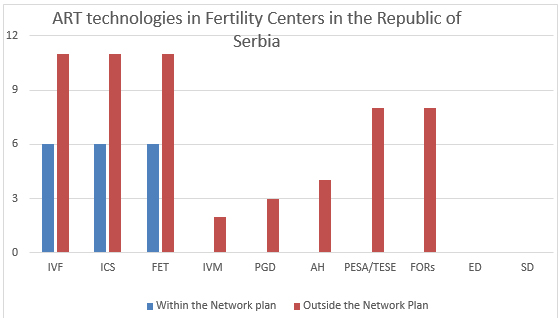
ART technologies funded by National health insurance fund include
in vitro fertilitation, intracytoplasmic sperm injection and frozen
embryo transfer. Patients whose medical indications require for some
other technology may approach Fertility Centers outside the Network
plan on their own budget (Chart 2).
Chart 2. ART technologies funded by National
health insurance fund and funded by patients
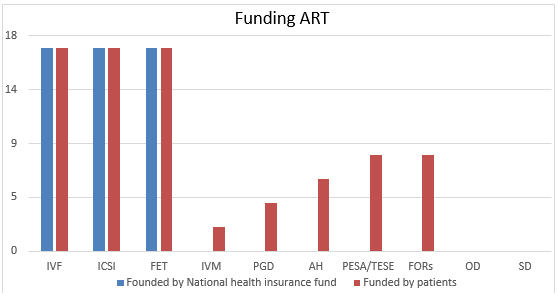
DISCUSSION
Additional challenges for couples in our country are weakness of
the so-called National System for the implementation of ART
procedures. Such problems are relatively numerous. They concern the
assembling necessary diagnostic analyses and documentation for
eligibility and criteria needed for the onset of the procedure.
Frequent inadequate equipment of institutions and expertise of staff
that provide services during the BMPO procedure, lack of application
of the most modern methods and procedures of reproductive medical
science, as well as the existence of a relatively long waiting
period for the procedure itself. The time dimension is extremely
important here, taking into account that the patient's age is
extremely important for the success of fertilization [13].
Earliest found data in the Republic of Serbia date from 2004, where
eight private clinics are mentioned and their internal documentation
could not be obtained. In Serbia, ART was only done on Clinic for
Gynecology and Obstetrics of the Clinical Centre of Serbia. Summary
annual reports show that the number of treatments varied and it
probable depends on the (under)development of technology, but also
on the social and economic factors. These data show that the number
of ART in public institutions, compared to the number of started
cycles in Serbia for the year of 2000, amounted to 178 started
cycles; 296 started cycles for the year of 2001; 174 started cycles
for the year 2002; and 149 started cycles for the year of 2003. A
very expensive ART procedure in Serbia was financed by couples
themselves [1].
National health insurance fund has financed infertility treatments
by Biomedical Assisted Fertilization procedures since 2006,
according to indications prescribed by Natinonal Expert Commission
of the Ministry of Health of the Republic of Serbia. Between 2009
and 2013, The Ministry of Health has passed the Law on the Treatment
of Infertility Procedures of Biomedical Assisted Fertilization
(Official Gazette of the Republic of Serbia”, No. 72/2009), as well
as a series of bylaws that regulate this area. As the existing
capacities of medical institutions within the Network plan are not
enough to meet the needs of all insured individuals, National health
insurance fund has concluded contracts for administering mentioned
services with private medical institutions on several occasions
[14].
In the year of 2013, there were 634 second phases of assisted IVF
fertilizations done in Serbia, accompanied by 1,105 ICSI procedures
[15]. Based on the data available from analyzed planned and achieved
scope of content rights of insured individuals to stationary medical
care in the Republic of Serbia in 2013, the right to infertility
treatment financed by the Fund (based on invoiced services of
National health insurance fund) was granted to 2055 patients, 1659
of which in Fertility Centers within the Network plan (925 IVF and
734 ICSI) and 396 in private BAF Centers outside the Network plan
mreže (25 IVF and 371 ICSI). Frozen embryo transfer was not financed
by the Fund, so patients financed the procedure themselves in
Fertility Centers outside the Network plan, of which there are no
accurate data.
The total of 933 second phases of assisted fertilizations by IVF
method were done in 106, followed by 1,474 ICSI methods and 140
frozen embryo transfers [11]. Based on the data available from
analyzed planned and achieved scope of content rights of insured
individuals to stationary medical care in the Republic of Serbia in
2016, the right to infertility treatment financed by the Fund (based
on invoiced services of National health insurance fund) was granted
to 2407 patients, 1,529 of which in Fertility Centers within the
Network plan (854 IVF and 675 ICSI) and 878 (79 IVF and 799 ICSI) in
private Fertility Centers outside the Network plan. Frozen embryo
transfer also came to be funded, so there were 140 invoices from
patients, 5 of which in Fertility Centers within the Network plan
and 135 in private ART centers.
There were 712 second phases of assisted fertilizations by IVF
method done in 2017, followed by 2,396 by ICSI method and 445 frozen
embryo transfers [12]. Based on the data available from analyzed
planned and achieved scope of content rights of insured individuals
to stationary medical care in the Republic of Serbia in 2017, the
right to infertility treatment financed by the Fund (based on
invoiced services of National health insurance fund) was granted to
4064 patients, 956 of which in Fertility Centers within the Network
plan (634 IVF and 322 ICSI) and 3,108 (712 IVF and 2,396 ICSI) in
private Fertility Centers outside the Network plan. Frozen embryo
transfer also came to be funded, so there were 445 patients, 5 of
which in Fertility Centers within the Network plan and 440 in
private Fertility Centers.
CONCLUSION
Based on the available and updated data we can conclude that
Fertility Centers in the Republic of Serbia have access to all the
important technologies for ART. Fertility centers within the Network
plan can implement only the technologies financed and invoiced by
the Fund. Based on the data available from analyzed planned and
achieved scope of content rights of insured individuals to
stationary medical care in the Republic of Serbia, it is evident
that the number of invoiced ICSI cycles is significantly larger in
Fertility Centers outside the Network plan, which shows that
patients with graver medical indications are referred to private
clinics. Thus for example patients with medical indications for
azoospermia do not have possibility of treatment in Fertility
centers within the Network plan, only in Fertility centers outside
the Network plan that are financed by patients themselves. Moreover,
the higher number of available ART technologies may be one of the
reasons for the significant growth of services provided in private
medical institutions.
P.S. A question arises as to why the lawfully granted oocyte and
sperm donation services are not available neither in Fertility
Centers within, nor outside the Network plan. If Fertility Centers
within the Network plan are not equipped, why aren’t these services
available in Fertility Centers outside the Network plan?
LITERATURE:
- Devedžić M. Development of reprogenetics and its demographic
aspects. BIBLID 0038-982X(2004): 1-4 p.45-65. Available from:
https://www.researchgate.net/publication/
275676887_Development_of_reprogenetics_and_its_demographic_aspects
- Begum MR. Assisted Reproductive Technology: Techniques and
Limitations. Journal of Bangladesh College of Physicians and
Surgeons Vol.26, No.3, September 2008. Available from:
https://www.researchgate.net/publication/
270114969_Assisted_Reproductive_Technology_Techniques_and_Limitations
- Keane M, Long J, O’Nolan G, Farraghe L. Assisted
reproductive technologies: International approaches to public
mechanisms and criteria. An evidence review. Health Research
Board: Dublin; 2017. Available from:
https://www.researchgate.net/publication/322244930_Assisted_reproductive_technologies
_International_approaches_to_public_funding_mechanisms_and_criteria_An_evidence_review
- WHO laboratory manual for the examination and processing of
human semen. 5th ed. Geneva: Switzerland; 2010.
- In vitro fertilization (IVF) & intra-cytoplasmic sperm
injection (ICSI) [homepage on internet]. New Zeland: Merck
Serono Australia ; 2011. Available from:
https://fertilityfirst.com.au/wp-content/uploads/2017/02/in-vitro-fertilisation-ivf-intr.pdf
- Bjelica A. Komparacija politika vantelesne oplodnje u Srbiji
I drugim evropskim zemljama. Timočki medicinski glasnik. 2017;
236-244. Available from:
http://www.tmg.org.rs/v420406.htm
- Grynberrg M, Hachem HE, de Bantel A, Benard J, Parco S,
Fanchin R,. In vitro maturation of oocytes: uncommon
indications. Fertility and Sterility. 2013; 99(5). Available
from:
https://www.fertstert.org/article/S0015-0282(13)00133-7/fulltext
- Calhaz-Jorge C, De Geyter C, Kupka MS, De Mouzon J, Erb K,
Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V. Assisted
reproductive technology in Europe, 2013: results generated from
European regiters by ESHRE. Human Reproduction 2012; 32(10).
Available from:
https://pubmed.ncbi.nlm.nih.gov/29117383/
- Shenfield F, De Mouzon J, Scaravelli G, Kupka M, Ferraretti
AP, Prados FJ, Goossens V. Oocyte and ovarian tissue
cryopreservation in European countries: statutory background,
practice, storage and use. Human Reproduction 2017; 1(003).
Available from:
https://academic.oup.com/hropen/article/2017/1/hox003/3092404
- Republički fond za zdravstveno osiguranje [homepage on
internet]:
http://www.rfzo.rs/index.php/osiguranalica/vto
- Brcanski J, Ločkić N, Živković Šulović M, Savković S.
Analiza planiranog i ostvarenog obima sadržaja prava osiguranih
lica na stacionarnu zdravstvenu zaštitu u Republici Srbiji u
2016.godini. Institut za javno zdravlje Srbije“ dr Milan
Jovanović Batut“. 2017. Available from:
http://www.batut.org.rs/download/izvestaji/AOIS 2016.pdf
- Brcanski J, Ločkić N, Živković Šulović M, Antanasijević D,
Savković S. Analiza planiranog i ostvarenog obima sadržaja prava
osiguranih lica na stacionarnu zdravstvenu zaštitu u Republici
Srbiji u 2017.godini. Institut za javno zdravlje Srbije“ dr
Milan Jovanović Batut“. 2018. Available from:
http://www.batut.org.rs/download/izvestaji/AIOS prava na
stacionarnu zdravstvenu zastitu.pdf
- Marjanski V. Mogućnost uvođenja privatnog osiguranja
troškova sprovođenja postupka biomedicinski potpomognutog
oplođenja (BMPO). Zbornik radova Pravnog fakulteta. 2012; 3:
297-308. Available from:
http://scindeks-clanci.ceon.rs/data/pdf/0550-2179/2012/0550-21791203297M.pdf
- Republički fond za zdravstveno osiguranje. Lečenje
neplodnosti postupcima biomedicinski potpomognutog oplođenja
preliminarni izveštaj. Available from:
http://www.rfzo.rs/download/vto/Preliminarni Izvestaj o lecenju
neplodnosti postupcima BMPO,010414.pdf
- Radović Crnčević Lj, Savković S, Mutavdžić T. Analiza
planiranog I ostvarenog obima I sadržaja prava osiguranih lica
na stacionarnu zdravstvenu zaštitu u Republici Srbiji u 2013.
Godini. Institut za javno zdravlje Srbije“ dr Milan Jovanović
Batut“. 2014. Available from:
http://www.batut.org.rs/download/izvestaji/2013AnalizaObimaISadrzajaStacionarna.pdf
|
|
|
|


