| |
|
|
INTRODUCTION
Nodules in the thyroid gland are very common and can be found in
50-68% of adults in the general population. Only about 5% of these
nodules are malignant and require treatment. They usually do not
give any discomfort. When they are discovered, they should be
assessed on the basis of clinical, echosonographic and cytological
findings, and, if necessary, using additional diagnostic methods,
and make a decision on the need for treatment. Based on the
ultrasound characteristics of the nodule, it is decided whether
further diagnosis is needed, in terms of thin needle aspiration
puncture (FNA) and cytological examination, after which a decision
is made on further procedure [1-5].
Currently, FNA is the most effective method for determining the
nature of the node. However, many nodules are benign, and even
malignant nodules, especially those smaller than 1 cm, often show
indolent and non-aggressive behavior. Therefore, not all detected
nodes require FNA. A reliable non-invasive method to detect nodes
indicated for FNA would be highly desirable [6]. Ultrasound is the
initial diagnostic method for the detection of thyroid nodules. In
addition to the presence of nodules, it accurately determines the
size, location and number of nodules in the thyroid gland (thyroid).
This non-invasive screening method is safe, harmless and can be
repeated [7]. Assessing the risk of malignancy is very important in
patients with glandular nodules in order to identify those nodules
that need to be punctured with a thin needle. The main disadvantage
of this examination is that it largely depends on the doctor
performing the examination [8]. Therefore, an attempt was made to
find a formula for risk assessment in relation to ultrasound
characteristics and standardization of ultrasound description, in
order to reduce the subjectivity of the examiner. Koike E. et al.
from the Noguchi Thyroid Clinic and Hospital Foundation from Japan
in 2001. set the formula for the prediction of thyroid nodule
malignancy based on 5 ultrasound characteristics of the nodule:
margins, shape, echogenicity, echostructure and calcification
[9,10]. As no characteristic can reliably predict malignancy, the
use and combination of several traits or characteristics is advised.
One such system, that is, a way of combining and scoring several
properties of a node in the thyroid gland, was published in 2009 by
Horvath et al. as the Thyroid Imaging Reporting and Data System (TIRADS).
It consists of a scale of 6 characteristics for stratification of
malignancy risk. Subsequently, similar recommendations were issued
by the Korean Thyroid Radiology Society, the American Thyroid
Association, the American Association of Clinical Endocrinologists,
the American College of Endocrinology, and the Italian Association
of Clinical Endocrinologists [8].
In 2015, the American College of Radiology (ACR) issued instructions
for access to the most common thyroid nodules and gave instructions
for standardizing the ultrasound examination of the thyroid gland.
Thyroid Imaging Reporting and Data System (ACR TI-RADS) [6].
Based on a review of the literature, the American Association of
Clinical Endocrinologists, the American Thyroid Association and
Korean guides, in 2017 a new EU-TI RADS (European Thyroid Imaging
Reporting and Data System) classification was formed to assess
thyroid nodules and decide on a possible FNA nodule [7]. .
In the following, the European Thyroid Imaging Reporting and Data
System (EU-TI RADS) and the American College of Radiology (ACR),
Thyroid Imaging Reporting and Data System (TI-RADS), ACR TI-RADS
will be described.
GUIDELINES FOR STANDARDIZATION OF ULTRASOUND
EXAMINATION OF THE THYROID GLAND EU-TI RADS
Category EU-TIRADS 1, is a category, ie thyroid gland
(thyroid) that does not contain nodules.
Benign category (EU-TIRADS 2), risk of malignancy close to
0%.
This category includes completely anechoic nodules (cysts) and
completely spongiform nodules.
Pure cystic changes, cysts, are characterized by the absence of wall
thickening, posterior signal amplification as well as the absence of
a solid component, regardless of their size. Figure 1.
Figure 1. Completely cystic nodule (From: Gilles
R. et all. European Thyroid Association Guidelines for Ultrasound
Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS.
Eur Thyroid J 2017; 6: 225–237.)
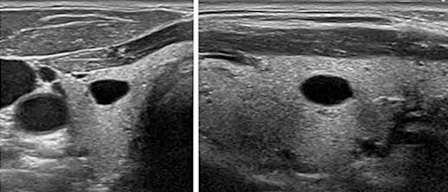
This category also includes cysts that are divided into separate
sections by fibrous septa. The presence of echogenic material within
the cyst is often encountered and may correspond to either a clot of
fibrin, a colloid, or a true solid component, which may be
distinguished by the use of Doppler. If there is a suspicion
regarding the existence of a solid component inside the cyst, such a
node should be classified as low risk. Spongiform nodules are
composed of tiny cystic spaces that cover the entire nodule, with
the size of the nodule not playing a role in assessing the risk of
malignancy. Small cystic spaces are separated by numerous isoechoic
septa. Figure 2. If cystic spaces do not fill the entire node, the
node should be classified as a low-risk node. Pure cystic changes
and completely spongy nodules should be considered benign. FNA is
not recommended for these changes, regardless of their size, and
even for such benign cystic nodules, ablation with ethanol is
recommended as the therapy of first choice [8,11].
Figure 2. Spongiform node. (From: Gilles R. et
all. European Thyroid Association Guidelines for Ultrasound
Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS.
Eur Thyroid J 2017; 6: 225–237)
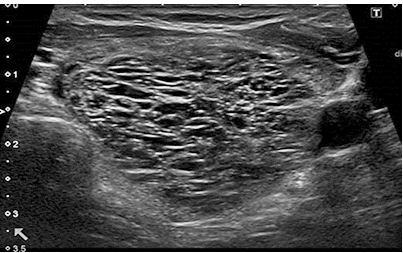
Low risk category (EU-TIRADS 3), where the risk of
malignancy is 2-4%.
These nodes are characterized by an oval shape, smooth edges
(margins), as far as echogenicity is concerned, these nodes are
isoechoic or hyperechoic, without any high-risk characteristics.
Figure 3, isoechoic nodule, Figure 4 hyperechoic nodule. Nodes with
these characteristics have a low risk of malignancy and FNA for
nodes> 20mm should be considered. The 20 mm threshold was chosen
based on the argument that distant metastases are rarely found in
follicular carcinomas <2 cm [12]. Grouped and associated nodes (polynodose
goiters) of these characteristics should be included in this
category, and FNA should be considered if one or more nodes are> 20
mm. It should be noted that a completely homogeneous isoechoic
nodule may correspond in less than 4% of cases to follicular
carcinoma or follicular variant PTC [13,14]. However, even minimal
cystic changes in the nodule favor benignity [15]. So oval-shaped
nodules, which are isoechoic or hyperechogenic with smooth margins
and without high-risk characteristics, should be classified as
low-risk. FNA is usually only recommended for nodes> 20 mm [8].
Figure 3. Isoechogenic nodule. (From: Gilles R. et
all. European Thyroid Association Guidelines for Ultrasound
Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS.
Eur Thyroid J 2017; 6: 225–237)
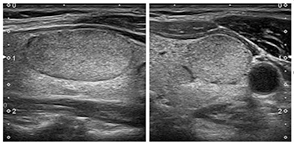
Figure 4. Hyperechogenic nodule. (From: Gilles R. et all. European
Thyroid Association Guidelines for Ultrasound Malignancy Risk
Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur
Thyroid J 2017; 6: 225–237)
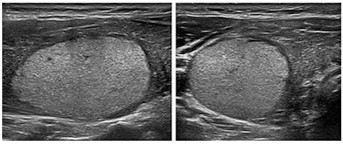
Medium risk category (EU-TIRADS 4) where the risk of
malignancy is 6–17%.
These nodules are characterized by an oval shape, smooth edges, mild
to moderate hypoechoicity, without other high-risk features. Figure
5. The difference between the low and medium risk category lies in
the echogenicity of the solid component of the node. In the case of
heterogeneous echogenicity of a solid component, the presence of any
hypoechoic change classifies the node into a medium-risk category.
The presence of a thin halo, partially cystic changes, comet-type
artifact, peripheral vascularity, reduce the risk of malignancy.
Hypoechoic nodes should be classified as moderate risk, including
those with cystic areas, bearing in mind that the risk is lower in
partially cystic nodes than in completely compact nodes.
Characteristics such as discontinuous peripheral margins, peripheral
macrocalcifications, dense halo, predominantly central
vascularization may increase the risk of malignancy. In this group,
the threshold for FNA is recommended for nodes larger than 15mm [8].
Figure 5. Hypoechogenic nodule. (From: Gilles R.
et all. European Thyroid Association Guidelines for Ultrasound
Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS.
Eur Thyroid J 2017; 6: 225–237)
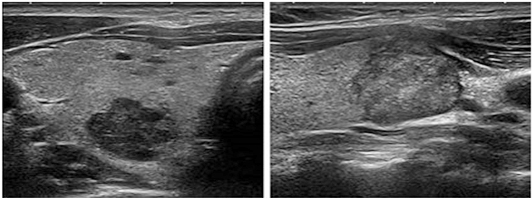
High risk category (EU-TIRADS 5), where the risk of
malignancy is 26–87% The characteristic of these nodes is the
presence of at least one of the following characteristics, which
belong to the characteristics (features) of high risk: not oval
shape (higher than wider), irregular edges, microcalcifications and
marked hypoechoicity. Figure 6.
Figure 6. Nodus from the high risk category.
(From: Gilles R. et all. European Thyroid Association Guidelines for
Ultrasound Malignancy Risk Stratification of Thyroid Nodules in
Adults: The EU-TIRADS. Eur Thyroid J 2017; 6: 225–237)
All these characteristics show high rates of specificity
(83–84%), but also low rates of sensitivity (26–59%). Pronounced
hypoechoicity has the lowest sensitivity of the four described
characteristics and is specific only if the node is compact, because
a highly hypoechoic node may be the remnant of a previous cyst. In a
partially cystic node, microcalcifications are the best predictor of
malignancy, while other features appear less significant. At the
same time, the presence of several anomalies, jagged intermittent,
edges with spicules, lobulations, dotted echogenic foci, non-oval
shape, increase the risk of malignancy. Nodes with such properties
that are larger than 10 mm should undergo FNA, except in inoperable
patients for any reason or a short lifespan is expected, due to the
existence of other comorbidities [8]. In case of a benign
cytological result, FNA of such a node, the puncture should be
repeated within 3 months in order to reduce the number of false
negative findings. In the case of nodules smaller than one
centimeter with high-risk ultrasound characteristics, it is
recommended to actively monitor the nodules as well as to treat
pathological lymph nodes in the neck and the symptoms and signs that
the patient himself reports. It is known that few or none of these
patients will develop distant metastases, ie that mortality is
negligible even if the nodule corresponds to cancer, in the case of
subsantimetric dimensions of the nodules [8]. Patients with
subcentimeter nodules and very suspicious ultrasound characteristics
but without abnormal lymph nodes in the neck should be presented
with the possibility of active surveillance as one option or FNA
under ultrasound control.
EU-TIRADS scoring is also useful in multinodular thyroid gland for
selecting nodes that are candidates for FNA. During the
echosonographic examination, ultrasound high-risk nodes should be
identified, observed, described, and FNA suggested if the node is
larger than 10 mm.
Identify medium risk nodes; describe only those nodes larger than 5
mm and for FNA suggest those larger than 15 mm. Identify low-risk
nodes; describe only those larger than 10 mm and suggest for FNA for
those larger than 20 mm. If there are more nodes, more than three,
describe in detail only those that are suspicious (according to the
previous risk and size criteria), record the others [8].
SIGNIFICANCE OF OTHER ULTRASOUND CHARACTERISTICS
Shape, margins, echogenicity, composition and microcalcifications
are the basic characteristics that enable EU TIRADS classification.
However, some of the ultrasound features can be used to further
assess and classify risks and modulate indications for FNA.
Suspicious lymphadenopathy
Ultrasound assessment of cervical lymph nodes should be performed in
all patients with thyroid nodules, especially in those with medium
and high risk.
In suspected lymph nodes, lymph node FNA should be performed for
cytological analysis as well as for determination of thyroglobulin
and calcitonin [8].
Extrathyroid propagation, proliferation and invasion of
surrounding tissue
Propagation into adjacent structures and disruption of thyroid
capsule continuity may be considered a specific feature for invasive
malignancy. Adherence to the capsule, ie close contact with the
capsule, has less specificity for macroscopic extrathyroid invasion
and spread, through the capsule. The presence of an unaltered
thyroid parenchyma, 2 mm between the nodule and the continuous,
compact thyroid capsule, indicates that there is almost no
macroscopic extrathyroid expansion and invasion while reducing the
risk of microscopic capsule invasion and extrathyroid expansion. The
discontinuity of the capsule, the adhesion of the capsule and the
bulge of the capsule must be emphasized in the report, ie the
ultrasound description, due to the possible invasion of the capsule
and extrathyroid expansion [8].
Macrocalcifications and hyperechoic points (foci)
Macrocalcificationscan be defined as echogenic foci (points) larger
than 1 mm with the existence of posterior shading (acoustic window).
- Isolated central intranodularmacrocalcifications are not
necessarily associated with malignancy, ie they do not
inevitably indicate malignancy.
- Isolated macrocalcification, which almost completely fills
the calcified node, has a low risk of malignancy.
- Calcifications on the periphery, peripheral calcifications
(peripheral or curvilinear) or (picture of broken egg shell)
along the periphery of the node, increase the risk of malignancy
if their continuity is interrupted [8].
Hyperechoic points (foci, spots)
These changes correspond to perimilimeter hyperechoic changes and
can be caused by:
- Colloidal crystals or remnants of fibrin that create
artifacts (reverberations), comet tails and are almost always a
sign of benign change.
- Posterior acoustic amplification (posterior, posterior wall
of the cyst, iemicrocystic area) is mainly seen in
high-frequency probes and is a feature that indicates benignity.
- True microcalcifications correspond to psammomic bodies
around which there are multiple round echogenic foci up to 1 mm
in size without the existence of posterior shading (acoustic
headlight) and they are always placed in a solid, homogeneous
component of the node. Microcalcifications largely suggest
malignancy.
- Hyperechogenic spots of indeterminate significance that
cannot be classified with certainty in the previous three
categories. Rather linear than round and without microcystic
cavities and comet tail artefacts.
Isolated macrocalcifications are not specific for malignancy.
Their presence should be correlated with other ultrasound
characteristics. Echogenic spots of comet tail appearance suggest
benignity. True microcalcificationsshould be distinguished from
other echogenic spots and such nodules should be subjected to FNA
[8].
Halo
The halo is thought to correspond to the nodule capsule or
surrounding blood vessels, or to sometimes correspond to the
surrounding compressed parenchyma. A thin halo reduces the risk of
malignancy (0.3mm), while a thick halo or absence of halo increases
the risk of malignancy. However, a clear definition of thin and
thick halo cannot be given [8].
Vascularization
As for vascularity, the description of vascularity with the help of
color Doppler is often used in clinical practice. Malignant nodules
are more likely to have type III vascularity, while benign nodules
show type I and II vascularity. Type I vascularity, denotes absent
or scarce vascularity. Type II, denotes present perinodal and scarce
intranodal vascularization and type III denotes scarce perinodal and
pronounced intranodal vascularization.
However, it is very important that the intranodular signal increases
with the size of the benign nodule. Vascularity as a criterion
remains for the assessment of nodules remains controversial, mainly
because the assessment of vascularity largely depends on the
equipment and settings of the ultrasound apparatus and because it
largely depends on the subjective assessment of the examiner.
Therefore, the ETA working group does not recommend the inclusion of
vascularity in the assessment in the TIRADS score [8].
Node growth
Regarding the growth of thyroid nodules, the published results
suggest that the growth of nodules cannot accurately distinguish
between benign and malignant lesions. Thus, determining the growth
of nodules is not recommended as a criterion for distinguishing
malignant and benign nodules [8].
The EU-TIRADS scoring system is based on the presence of ultrasound
characteristics that are highly suspected of malignancy. This system
includes five categories, ultrasound findings. The first category
involves the absence of thyroid nodules, the other four include
benign, low-suspicion, moderate-suspicion, and high-suspicion
categories. Compared to other risk scoring systems, the main
advantage of EU-TIRADS is the facilitation of scoring in the use of
specific ultrasound features to detect high-sensitivity thyroid
cancer which should allow for the reduction of unnecessary FNA
procedures [8].
Very few nodules will require invasive processing that includes
cytology and molecular testing (FNA). An ultrasound examination with
an assessment of clinical risk factors will be sufficient for an
initial monitoring and diagnostic strategy. This is especially
important for weak, elderly people, with comorbidities, because they
are unlikely to be endangered by the thyroid tumor itself, and
excessive diagnosis and interventions can do more harm than good.
The goal is to identify the best strategy for the individual in
terms of disease outcome and quality of life, avoiding the pitfalls
of over-diagnosis and over-treatment [16].
ACR TI-RADS
THYROID IMAGING REPORTING AND DATA SYSTEM ACR TI-RADS
In this system, when evaluating the node, it is necessary to
determine (score) each of the characteristics or ultrasonic
properties of the node, which will be listed later, after which
points are added. The total number of points determines the level of
ACR TI-RADS score, which ranges from TR1 which is benign to TR5
which is a highly suspicious finding for malignancy. Recommendations
for FNA and ultrasound monitoring of the node are based on the level
of the number of points and its maximum diameter. Ultrasound,
characteristics or properties to be scored are the composition of
the node (composition), the echogenicity of the node, the shape of
the node, the margins or edges of the node and the echogenic points
or foci [6].
Composition
Nodes that are cystic or almost completely cystic do not bring any
points, because they are almost always benign. Similarly, spongy
material is highly associated with benign characteristics,
regardless of other characteristics. However, the spongy node must
be composed of at least 50% of small cystic spaces. Nodes should not
be characterized as spongy only on the basis of the presence of
several scattered cystic elements in a solid node. Mixed cystic
solid nodules are categorized as predominantly solid and
predominantly cystic. A solid component that is eccentrically placed
and has a sharp angle in relation to the wall of the node is
suspicious as well as a solid component that is hypoechoic, with
lobulations and point echogenic foci. Completely cystic,
predominantly cystic and spongy nodes are scored from zero points.
Mixed, cystically solid nodes are scored with one point, and
predominantly, ie mostly solid with two points [6].
Echogenicity
This feature refers to the reflectivity of the nodule in relation to
the surrounding thyroid tissue, except for very hypoechoic nodules
where muscles attached to the hyoid bone are used as a basis for
comparing echogenicity. This category also includes anechoic changes
with zero points, which refers to cystic or almost cystic nodes, and
extremely hypoechoic nodes to which three points would be awarded
due to their very hypoechoic picture. Anechoic nodes get zero
points, isoechoic and hyperechoic one point, and hypoechoic two
points, while highly hypoechoic nodes get three points [6].
Shape
Higher than wider (ovoid) is a non-sensitive but highly specific
indicator of malignancy. This property is estimated in the axial
plane by comparing the height and width of the node measured
horizontally and vertically in the transverse section. A higher than
broader configuration is usually obvious and rarely requires formal
measurements. This shape got three points, the oval shape zero
points [6].
Edges
Smooth and clear edges of the node reduce the risk of malignancy,
the edges (margins of the node) with such characteristics get zero
points. For nodes where we cannot estimate the edges, we classify
them in the category of nodes with a poorly defined edge of the
node, and that category gets zero points. A lobed or irregular
margin refers to a serrated or needle-like edge, with or without
protrusions in the surrounding parenchyma, and this characteristic
of the node is scored with two points. Propagation beyond the
thyroid gland is classified as extensive or minimal and is scored
with three points. Extensive extrathyroid spread, which is
characterized by invasion of the surrounding soft tissue or vascular
structures, is a highly reliable sign of malignancy and is one of
the unfavorable prognostic signs. Minimal invasion may be
echosonographically suspicious if we have little thyroid parenchyma
between the nodule and the thyroid capsule or there is swelling
(bulge) of the contours and loss of echogenicity of the thyroid
border [6].
Echogenic foci
The comet's tail artifact is an echogenic focus with V-shaped echoes
whose depth is greater than 1mm. They are found in cystic components
and are characteristic of benignity, so that for this characteristic
the node received zero points. Macrocalcifications are rough
echogenic foci accompanied by acoustic shadows. For their existence,
one point was awarded. Peripheral calcifications located along the
entire margin or along one part of the margin receive two points.
Some authors have drawn attention to intermittent peripheral
calcifications with bulging soft tissue, as suspected malignancies.
For nodes with calcifications that cause a strong acoustic shadow
that prevents or limits the assessment of internal characteristics,
especially echogenicity and composition, it is best to assume that
the node is solid and assign 2 points for composition and one point
for echogenicity [6].
Point (punctiform) echogenic foci are smaller than
macrocalcifications and they are without acoustic shadow. For their
existence, the node received three points. In solid constituents of
thyroid nodules, they may correspond to psammomatous bodies
(calcifications) that are associated with papillary carcinomas, and
are therefore considered highly suspicious, especially in
combination with other suspicious properties. This category includes
echogenic foci that are associated with small comet tail artifacts
in solid node components, as opposed to the large comet tail
artifacts listed earlier. Significantly, small echogenic foci can be
seen in spongy nodules, where they probably represent the posterior
walls of small cysts. They are not suspicious in this case and
should not be given any points [6].
Additional benign phenomena
Several ultrasound findings have been described as characteristic of
benign changes with a high degree of reliability. These findings
include the existence of uniform hyperechogenicity (white knight),
as well as the variegated appearance of hyperechogenic areas,
divided by hypoechoic bands resembling giraffe skin, both present in
Hashimoto's thyroiditis.
Node size as an indication for FNA
In a 2005 publication, Machens et al. [17] reported that the
cumulative risk for distant metastases for papillary and follicular
thyroid cancers increased significantly for nodules larger than 2
cm. So he suggested a biopsy of nodules larger than 2 cm. Machens et
al. Based their analysis on tumor size in resected samples rather
than ultrasound. Subsequent studies have shown a significant lack of
concordance between sonographic and pathohistological sizing, with
the tendency of ultrasound toresult in larger measurements [18].
ACR TI-RADS is in accordance with most other guidelines in the
recommended FNA for highly suspicious nodes of 1 cm or larger. That
is, for slightly suspicious and moderately suspicious nodules larger
than 2.5 and 1.5 cm. Biopsy is usually not indicated in a gland that
is interspersed with multiple confluent nodules of similar
characteristics [6].
Ultrasound report
For the ultrasound report, the exact dimension of the thyroid
nodules is very important, since the maximum dimension of the nodule
determines whether a given node should be biopsied or monitored.
Nodes should be measured in three planes. Maximum dimension in axial
projection, maximum dimension in perpendicular projection in
relation to the previous measurement, maximum longitudinal dimension
in sagittal plane. The measurement should include a halo node if
present. A calculation can also be used, which determines the
volume. In addition to the dimensions, it is necessary to describe
the ultrasonic characteristics, previously listed, on the basis of
which the scoring is performed. It should be described whether the
node touches the trachea or whether it is close to the
tracheoesophageal groove (the place of the recurrent laryngeal
nerve). An accurate description of the location of the nodes on the
sonograms is equally important, especially when the gland is
heteroechoic or multiple nodes are present. In the polynodose gland,
describe accurately and in detail only the nodes that meet the
criteria for FNA, only the others. As far as FNA is concerned, a
biopsy of more than two nodes is not recommended, puncturing the
most successful nodes. The decision to repeat a biopsy is usually
made by physicians who monitor the patient based on previous FNA
results from the Bethesda system for thyroid cytopathology [18].
Definition of growth
Criteria for significant growth depend on the size of the node,
which must also take into account the variability of measurements.
Significant magnification is defined as a 20% increase in at least
two node dimensions and a minimum increase of 2mm, or a 50% or
greater volume increase [6].
Tracking time
There is little agreement in the literature about the optimal time
to monitor nodules, since the degree of growth does not reliably
distinguish benign from malignant nodules. Examination intervals
shorter than one year are not recommended, except for proven
malignancies under active supervision, which may require more
frequent monitoring. It is advisable to determine the monitoring
intervals in relation to the number of points assigned to the node.
For a TR5 lesion, we recommend monitoring once a year for 5 years.
The first, second, third and fifth years should be done for TR4
controls. For TR3 controls can be performed in the first, third and
fifth years.
Monitoring can be stopped after five years, if there are no changes
in size, because stability in this time interval reliably indicates
that the node behaves benignly, which is valid for all categories of
nodes [6].
There are no published data for the treatment of nodules that
increase significantly, if their size is still below the threshold
for FNA and remain in the same number of ACR TI-RADS points for
almost five years, but their monitoring is still necessary. If the
ACR of the TI-RADS node increases during monitoring, the next
control should be done in one year, regardless of its initial level
[6].
Assessment of cervical lymph nodes
The suspicious finding is suggestive in spherical lymph glands,
hyperechoic glands, loss of normal echogenic hilus, presence of more
pronounced peripheral flow or vascularization than hilus.
Heteroechogenicity with cystic components and point echogenic foci
that may represent microcalcificationsis also a suspicious finding
[6].
Categorization (scoring) of nodes after scoring
After scoring the ultrasonic properties of the nodes, the nodes are
categorized as, TR1-TR5. TR1, benign nodules with 0 points TR2,
non-suspicious nodes with 2 points, where FNA is not recommended for
these nodes, TR3 minimally suspicious nodes with 3 points, where FNA
is advised for nodes larger than 2.5 cm and for nodes larger than
1.5cm tracking, TR4 moderately suspicious nodes with 4-6 points,
with FNA recommended for nodes larger than 1.5cm and for nodes
larger than 1cm tracking and TR5 highly suspicious nodes having more
than 7 points, with FNA is advised for nodes larger than 1 cm and
monitoring for larger than 0.5 cm [6].
In addition to the ultrasound appearance of the nodule, other
factors must be taken into account when deciding on FNA. TSH should
be measured in all patients to rule out the possibility of a
hyperfunctional node. Such lesions do not require a biopsy, because
they are practically always benign. Risk factors for malignancy are
exposure to ionizing radiation during childhood accidentally or for
medical reasons, positive family history of thyroid malignancy,
occurrence of nodules in children and the elderly, clinical
features, nodules that are firm, hard, fixed to the substrate and
the environment, grow rapidly. It has recently been confirmed that
node location is also an independent risk factor for malignancy.
Nodes located in the isthmus carry a higher risk of malignancy,
while those located in the lower third of the lobe carry the lowest
risk compared to those from the middle or upper lobes. These factors
are not usually classified as a stratification algorithm, but may
influence a definitive attitude in joint decision-making with
patients about further diagnostic and therapeutic procedures [19].
Conclusion
Certain ultrasound properties, the characteristics of nodules in
the thyroid gland, can significantly indicate malignancy and are
used as criteria for FNA. The features with the greatest diagnostic
significance for predicting malignancy are the shape of the nodule,
higher than wider in the transverse section, ie ovoid appearance,
the presence of small calcifications in the nodule, irregular
margins, while the spongy and cystic appearance of the nodule and
the presence of halo around the nodule significantly indicate
benignity. Node size is an unreliable parameter for estimating
nodes. These ultrasound properties have different sensitivity and
specificity, but unfortunately none of them is enough for certain
rejection or confirmation of malignancy. FNA is a very important
diagnostic method, but its performance must be selective since
systematic puncture of all nodes, regardless of size or appearance,
is not recommended. It is important that the indications for FNA be
based on clinical characteristics, as well as on echosonographic
stratification of the risk of malignancy.
REFERENCES:
- Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs
L, at al.; American Association Of Clinical Endocrinologists,
American College Of Endocrinology, And Associazione Medici
Endocrinologi Medical Guidelines For Clinical Practice For The
Diagnosis And Management Of Thyroid Nodules - 2016 UPDATE.
Endocr Pract. 2016;22(5):622-39. doi: 10.4158/EP161208.GL.
- Liénart F.Thyroid nodule: benign or malignant? Rev Med Brux.
2012;33(4):254-62.
- Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras
M, Cibas ES, Orcutt J, Moore FD Jr, Larsen PR, Marqusee E,
Alexander EK. Prevalence and distribution of carcinoma in
patients with solitary and multiple thyroid nodules on
sonography. J Clin Endocrinol Metab. 2006;91(9):3411-7.
- Gharib H, Papini E. Thyroid nodules: clinical importance,
assessment, and treatment. Endocrinol Metab Clin North Am.
2007;36:707-735.
- Hegedüs L. Clinical practice. The thyroid nodule. N Engl J
Med. 2004;351:1764-1771.
- Franklin N. Tessler, MD, CMa , William D. Middleton, MDb, at
al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS):
White Paper of the ACR TI-RADS Committee Franklin N. Tessler,
MD, CMa , William D. Middleton, MDb , Edward G. Grant, M. J Am
Coll Radiol 2017;14:587-595.
- Merima R. Goran. Značaj određivanja prediktivnih faktora za
prisustvo limfonodalnih metastaza kod papilarnog tiroidnog
mikrokarcinoma. Doktorska disertacija. Univerzitet u Beogradu,
Medicinski fakultet 2018. Beograd.
- Gilles Russa Steen J. Bonnemab Murat Faik Erdoganc Cosimo
Duranted Rose Ngue Laurence Leenhardta aThyroid and Endocrine
Tumors, Institute of Endocrinology, Pitié Salpêtrière H.
European Thyroid Association Guidelines for Ultrasound
Malignancy Risk Stratification of Thyroid Nodules in Adults: The
EU-TIRADS. Eur Thyroid J 2017;6:225–237
- Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A,
Kawamoto H, et al. Ultrasonographic characteristics of thyroid
nodules: prediction of malignancy. Arch Surg. 2001;
136(3):334-7.
- Oh EM, Chung YS, Song WJ, Lee YD. The pattern and
significance of the calcifications of papillary thyroid
microcarcinoma presented in preoperative neck ultrasonography.
Ann Surg Treat Res. 2014; 86(3):115-21.
- Enrico P, Herve M, Andrea F, Laszlo H. 2020 European Thyroid
Association Clinical Practice Guideline for the Use of
Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J
2020;9:172–185.
- Machens A, Holzhausen HJ, Dralle H: The prognostic value of
primary tumor size in papillary and follicular thyroid
carcinoma. Cancer 2005; 103:2269–2273.
- Yoon JH, Kim EK, Hong SW, Kwak JY, Kim MJ: Sonographic
features of the follicular variant of papillary thyroid
carcinoma. J Ultrasound Med 2008; 27:1431–1437.
- Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, Sohn CH,
Choi YH: Sonographic features of follicular variant papillary
thyroid carcinomas in comparison with conventional papillary
thyroid carcinomas. J Ultrasound Med 2009;28(12):1685-92.
- Na DG, Kim JH, Kim DS, Kim SJ: Thyroid nodules with minimal
cystic changes have a low risk of malignancy. Ultrasonography
2016; 35:153–158.
- Giorgio Grani, Marialuisa Sponziello, Valeria Pecce, Valeria
Ramundo, and Cosimo Durante. Contemporary Thyroid Nodule
Evaluation and Management. J Clin Endocrinol Metab, 2020;
105(9):2869–2883.
- Machens A, Holzhausen HJ, Dralle H. The prognostic value of
primary tumor size in papillary and follicular thyroid
carcinoma. Cancer 2005;103:2269-73.
- Deveci MS, Deveci G, LiVolsi VA, Gupta PK, Baloch ZW.
Concordance between thyroid nodule sizes measured by ultrasound
and gross pathology examination: effect on patient management.
Diagn Cytopathol 2007;35:579-83.
- Giorgio G, Marialuisa S, Valeria P, Valeria R, Cosimo D.
Contemporary Thyroid Nodule Evaluation and Management. J Clin
Endocrinol Metab, September 2020; 105(9):2869–2883.
|
|
|
|






