| |
|
|
Sensitive groups and comorbidities The lethality of
COVID-19 is in correlation with the age of the patient, the
lethality among younger individuals is much lower compared to the
relatively higher lethality among the elderly (1, 2, 3). People
above the age of 65, who also have comorbidities (hypertension,
diabetes, obesity, kidney and cardiovascular issues), are especially
at risk. Most people who pass away due to COVID-19 have had
underlying health issues (hypertension, diabetes and heart disease)
(4). Based on the data from the month of March in the United States
89% of those hospitalized due to COVID-19 have had underlying issues
(5). The Italian Superiore de Sanita institute reported that of the
patients that died due to COVID-19, the ones that had medical
documents, 96.1% had at least one comorbidity and the average amount
of comorbidities per patient was 3 to 4 (6). Based on this report
the most common underlying issues were: hypertension (66% of cases),
type 2 diabetes (29.8% cases), heart disease (27.6% cases), atrial
fibrillation (23.1% cases) and chronic kidney disease (20.2% cases).
Hypertension is closely linked with a more severe case of COVID-19
and is one of the most common underlying issues in patients with
severe pneumonia due to COVID-19 (7, 8, 9, 10, 11). Obese is also
linked with more severe COVID-19 cases (12, 13, 14). Also there is a
difference in the amount of severe cases between genders, men are
more likely to suffer from severe COVID-19. Early epidemiological
data from China and Italy have shown a higher death rate in men (15,
16, 17). In addition, there is the fact that in Europe 57% of those
infected with SARS-CoV-2 were men while 72% of fatal cases were also
men (18).
Reduced expression of ACE2 receptor in sensitive groups
Scientists have discovered that ACE2 expression in 30 different
tissues, collected from thousands of different patients, drops
significantly after the age of 60 (19). This correlates perfectly
with what is seen in the field, children and the young often have
mild symptoms while the elderly have overall more severe symptoms
due to COVID-19. The same scientists that had discovered the reduced
expression of ACE2 receptors in the elderly also discovered that
diabetics, especially those with type 2 diabetes, also have reduced
ACE2 expression. Also, a reduction in ACE2 expression in the
glomeruli and tubules of the nephrons was found in those affected by
type 2 diabetes and chronic kidney disease (20).
ACE2 expression is high in the endothelium of the heart and kidney.
Expression of ACE2 is significantly reduced in spontaneously
hypertensive rats. What we can conclude from this is the fact that
ACE2 expression is reduced in hypertension (21).
In animal models it has been shown that ACE2 expression drops off
with age and is also lower in males compared to females. This gives
us an explanation as to why we see much more severe COVID-19 cases
and COVID-19 related complications in older males (22, 23).
As we have mentioned above people with hypertension are especially
at risk of developing severe issues due to COVID-19, due to the fact
that in hypertension the expression of ACE2 receptors is below a
certain threshold (24).
Chaudhry F, Lavandero S, Xie X, et al. have created a great
theoretical model which illustrates that a reduction in ACE2
expression below a certain threshold is needed in order for severe
lung damage to occur and potential lung fibrosis to begin (25)
(Figure1.).
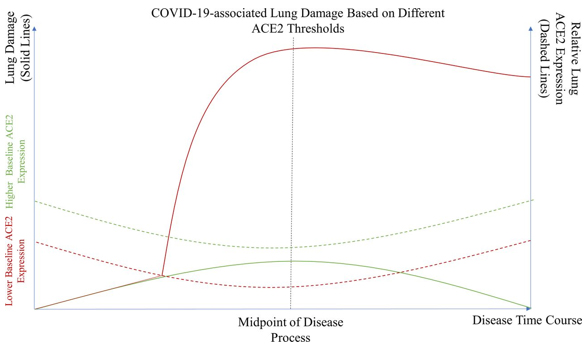
Figure 1. This is a theoretical model that
illustrates the severity of lung damage in patients with normal ACE2
expression (full green line) as well as in patient with reduced ACE2
expression that is mainly associated with comorbidities or risk
factors (full red line). Patient with a higher base level of ACE2
expression never fall below the critical level of ACE2 expression
(green dotted line) and are far less likely to suffer from severe
lung damage. On the other hand patients with a lower base level of
ACE2 expression (red dotted line) are far more likely to suffer from
severe lung damage.
The role of the ACE2 receptor from the aspect of the pathological
mechanism behind the action of the SARS-CoV-2 virus
When it comes to COVID-19 the one thing in common with all organs
affected by this disease is the fact that these organs have “large
functional surfaces” that need to be protected from an unregulated
immune response. The lungs are the best example for an organ such as
this (the alveoli and bronchi have a large functional surface). The
ACE2 receptors are primarily expressed on the club cells of the
bronchiole and on type 2 pneumocytes of the alveoli. Both of these
cells protect the lungs and help prevent the onset of Acute
Respiratory Distress Syndrome (ARDS). Club cells secrete a solution
that is similar to surfactant as well as other proteins which
protect the airways from severe inflammatory response by the immune
system. While type 2 pneumocytes defend the alveoli by secreting and
recycling the surfactant which is necessary to maintain normal
surface tension of the alveoli (26). The ACE2 receptors are far more
expressed on the cells of the lower airways than on the cells of the
upper airways (27).
Single cell RNA sequencing has revealed that the ACE2 receptor is
especially expressed on the surface of type 2 pneumocytes in the
alveoli. But, the ACE2 receptor is also expressed in organs that
have a “large functional surface”, such as: the kidneys (proximal
tubule cells), myocardium cells, the enterocytes which line the
ileum, the cells in the esophagus and the uroepithelial cells of the
bladder (28). From the facts above we can conclude that the ACE2
receptor has a protective role in the organs that have “large
functional surfaces”, the ACE2 receptor protects these surfaces from
an inflammatory response by the immune system.
The fact that ARDS occurs in severe COVID-19 cases only supports the
fact that the ACE2 receptor has a protective role. Lung biopsies
have unveiled severe inflammation and edema, which directly
corresponds with animal models in which lowered ACE2 expression is
associated with severe lung damage (29, 30). Those who had severe
H5N1 influence infections had similar findings (overreaction of the
immune system and a large amount of cytokine production) (31).
The molecular function of the ACE2 receptor on the cell
ACE2 (Angiotensin converting enzyme 2) belongs to a family of
angiotensin converting enzymes also known as dipeptidyl
carboxypeptidase. Besides the functions mentioned above ACE2 also
converts angiotensin 1 to angiotensin 1-9 and it also converts the
vasoconstrictive angiotensin 2 into the vasodilative angiotensin
1-7. ACE 2 is highly important in regulating the
renin-angiotensin-aldosterone which in itself is necessary in
maintaining optimal blood volume, blood pressure and as such is
crucial in the normal functioning of the cardiovascular system (32,
33).
Furthermore the ACE2 receptor also removes the C terminal remains of
many vasoactive peptides such as: neurotensin, kinetenin and des-Arg
bradykinin (34, 35). Besides these vasoactive peptides ACE2 also
catalyzes the breakdown of casomorphins, dynophrin A and apelins
(35, 36) .The ACE2 receptor is also very important for the transfer
of neutral amino acids through the gut lining (37).
Expression of ACE2 receptors is especially characteristic for the
following cells:
-alveolar type 2 cells (38, 39, 40)
-endothelial cells of both small and large arteries, and the smooth
muscle cells of the arteries (41)
-enterocytes of the small intestine, Leydig and Sertoli cells (41)
-proximal cells of the renal tubules and intestine cells (37)
-heart, kidney, testicle, and the gastrointestinal tract (34, 42,
43, 44, 45, 46)
A common characteristic of these organs, tissues and cells, which
have a high ACE2 expression, is that they have a “large functional
surface”. These are large cellular surfaces which are critical for
the normal function of the organs mentioned above. From this point
of a view we can conclude that the ACE2 receptor is critical in
maintaining the integrity and stability of these so called “large
functional surfaces”. We can also conclude that the ACE2 receptor is
important in the production of surfactant in order for the ACE2
receptor to realize its protective function.
The lungs have an especially large functional surface. In the lungs
the ACE2 receptor is located on the surface of alveolar type 2 cells
(AE2). It is a known fact that these cells are highly important in
the production of surfactant. Also it is known that the main role of
surfactant is to reduce the surface tension of alveoli and prevent
their collapse. Our findings however suggest that the cells that
produce surfactant, also have an immunomodulatory role (47).
The connection between acute lung injury, surfactant production, the
ACE2 receptor and an overreactive inflammatory response is best
displayed in experiments done on rats. Reduced ACE2 expression is
directly correlated with reduced production of surfactant (48).
Many authors have noted and recognized the important role that
surfactant and the ACE2 receptor have in protecting the lungs, as
well as the use of naturally and synthetically produced surfactant
in the treatment of COVID-19 patients (49).
The SARS-CoV-2 virus inhibits the production of surfactant by firmly
binding with the ACE2 receptor
As we have mentioned above the SARS-CoV-2 virus differs from other
SARS viruses by having a novel mutation in the S protein which
grants it the ability to firmly bind to the ACE2 receptor in order
to enter the targeted cell. By firmly binding to the ACE2 receptor
the virus has gained another mechanism by which it can damage the
host, and that mechanism is the inactivation of the ACE2 receptor
via intense binding with said receptor. We can confirm this by
observing people who inherited dysfunctional ACE2 receptors. These
people commonly suffer from Severe Acute Respiratory Distress
syndrome which mirrors severe COVID-19 cases. Besides COVDI-19,
dysfunctional ACE2 receptors are also associated with: hypertension,
kidney disease, myocardial infarction, type 2 diabetes and blood
vessel disorders (56). These are all comorbidities that are
associated with severe COVID-19 cases, and due to these factors it
is necessary that we begin researching the role that the ACE2
receptor has in producing surfactant in those with comorbidities.
The SARS-CoV-2 virus can inhibit the production of surfactant by
firmly binding with the ACE2 receptor.
The over reactive immune inflammatory response in the lungs seen
during SARS and ARDS that is caused by a lowered production of
surfactant is in itself sometimes caused due to a mutation in the
following genes necessary for surfactant production: SFTPA, SFTPB,
SFTBC, SFTBD, SFTA2, SFTA3. Mutations in these genes cause similar
consequences to those seen in ACE2 receptor inhibition via the
SARS-CoV-2 virus. The consequences we are talking about are
Respiratory Distress Syndrome in Premature infants, Pulmonary
Fibrosis and Interstitial pneumonia (51, 52, 53, 54, 55).
The conclusion from the information mentioned above is that no
matter the cause of ACE2 receptor inhibition (via the SARS-CoV-2
virus or via mutation) or surfactant production inhibition the end
result is the same when it comes to lung damage. Also other organs
are affected.
We can safely say that in order for the virus to achieve its
pathological potential on the molecular level it is necessary for it
to inhibit surfactant production and to disrupt the surface tension
of the alveoli.
Surfactants role in the stabilization and immunoprotection of large
functional surfaces
When it comes to so called “large functional surfaces” the role of
surfactant is two-fold. Its first role is in the reduction of
surface tension and its second role is in the protection of these
surfaces from the immune system.
These are two very important and broad functions. Surfactant has to
ensure that these large surfaces remain functional while at the same
time it has to protect these large surfaces and their antigen
specificities from systemic immunity.. Surfactant has a very gentile
molecular structure and as such is easily changed, which can have
systemic consequences. As mentioned above, organs and tissues which
have and need large functioning surfaces are: small blood vessels,
the alveoli of the lungs, the tubules and nephrons of the kidneys,
the GI tract and even blood platelets.
The major piece of evidence in the case that the SARS-CoV-2 virus
affects the lungs via blocking the ACE2 receptor and subsequent
surfactant production are the groups most affected by COVID-19. The
groups mostly affected are: people with hypertension, cardiovascular
patients, obstructive lung disease patients, people with chronic
kidney disease, the elderly (due to reduced elasticity in the blood
vessels and airways). All of the diseases mentioned in this
paragraph have one thing in common and that is the fact that the
large functional surfaces are damaged and not functioning optimally.
Surfactant is important on all large functioning surfaces. This is
especially the case when it comes to the lungs (57, 58), where large
surfaces are needed to facilitate the interaction between the air
that is inhaled and the circulating blood (59, 60). These surfaces
are vulnerable to and needs to be protected from an inflammatory
immune response (61, 62). A local inflammatory response here can
quickly escalate to a large systemic inflammatory response. When
such an inflammatory response escalates it quickly evolves into SARS.
The molecular mechanism of the pathological action of the SARS-CoV-2
virus as explained from the aspect of ACE2 binding and the
subsequent inhibition of surfactant production
After infection by the SARS-CoV-2 virus a literal race against the
clock begins between the rate of viral replication and the immune
system response to the virus. This is a race against the clock in
which the goal is to remain at a normal level of surfactant
production.
The virus needs the ACE2 receptor in order to enter the cell. When
the virus binds to the ACE2 receptor, the production of surfactant
is inhibited. The more viral particles there bind to this receptor
the less surfactant is synthetized.
If the infected person has a large amount of ACE2 receptors then the
amount of viral particles needed in order to reduce this number
below a critical threshold (as mentioned in the beginning of this
article) is also increased. This gives the immune system enough time
to effectively counteract the SARS-CoV-2 virus. That is why people
that are young, healthy, physically active and females are much more
resistant to COVID-19, due to the increased amount of ACE2 receptor
(the full green line mentioned in the beginning of this article).
People with a lower number of ACE2 receptors (the obese, elderly,
people with comorbidities, males) are more susceptible to the virus
occupying all avalaible ACE2 receptors and blocking the production
of surfactant to such a degree that the antigens of „large
functional surfaces“ become visible to the immune system and cause a
massive inflammatory response that we see in ARDS and SARS (the red
line in the picture in the begining of this article). The lungs are
usually the first site of this inflammatory response due to the fact
that surfactant is critical for their normal function. This is
basically a kind of inflammatory autoimmune response. This is
confirmed by the fact that immunosuppressive therapy, which is
normally used to treat autoimmune disorders, has thus far been
highly effective in the treatment of severe COVID-19 cases.
Take into consideration the lowered production of surfactant due to
ACE2 receptor inactivation. By implementing simple lifestyle changes
such as: physical activity and diet, the stability of surfactant
production can be improved. Also the role which vitamin D has in the
increase of ACE2 receptor expression, especially in sensitive
groups, must be taken into consideration when it comes to using
vitamin D supplementation as a preventative and protective measure
in sensitive groups. The knowledge gained by studying surfactant
should be used in the treatment of chronic diseases such as:
hypertension, cardiovascular diseases and others.
REFERENCE:
- Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith
B. "COVID-19-associated Acute Hemorrhagic Necrotizing
Encephalopathy: Imaging Features". Radiology. 2020; 296 (2):
E119–E120. doi:10.1148/radiol.2020201187. PMC 7233386. PMID
32228363.
- "Living with Covid19". NIH Themed Review. National Institute
for Health Research. 15 October 2020.
doi:10.3310/themedreview_41169.
- "How long does COVID-19 last?". UK COVID Symptom Study. 6
June 2020. Retrieved 15 October 2020. Available from:
https://covid.joinzoe.com/post/covid-long-term
- "The Epidemiological Characteristics of an Outbreak of 2019
Novel Coronavirus Diseases (COVID-19)". China CDC Weekly.
2020;2(8):113–122. doi:10.46234/ccdcw2020.032. ISSN 2096-7071.
Retrieved 15 June 2020.
- Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al.
"Prevalence and severity of corona virus disease 2019
(COVID-19): A systematic review and meta-analysis". Journal of
Clinical Virology. 2020;127: 104371.
doi:10.1016/j.jcv.2020.104371. PMC 7195434. PMID 32315817.
- Wang Y, Wang Y, Chen Y, Qin Q. "Unique epidemiological and
clinical features of the emerging 2019 novel coronavirus
pneumonia (COVID-19) implicate special control measures".
Journal of Medical Virology. 2020;92(6):568-576.
doi:10.1002/jmv.25748. PMC 7228347. PMID 32134116.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with
acute respiratory distress syndrome and death in patients with
coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern
Med 2020;180(7):934-943. doi: 10.1001/jamainternmed.2020.0994.
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities
in the novel Wuhan coronavirus (COVID-19) infection: a
systematic review and meta-analysis. Int J Infect Dis.
2020;94:91-95. doi: 10.1016/j.ijid.2020.03.017.
- Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics
of 140 patients infected with SARS-CoV-2 in Wuhan, China.
Allergy 2020;75:1730–41.
- Peng YD, Meng K, Guan HQ, et al. [Clinical characteristics
and outcomes of 112 cardiovascular disease patients infected by
2019- nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:E004.
- Emami A, Javanmardi F, Pirbonyeh N, et al. Prevalence of
underlying diseases in hospitalized patients with COVID-19: a
systematic review and meta-analysis. Arch Acad Emerg Med
2020;8:e35.
- Henry BM. "COVID-19, ECMO, and lymphopenia: a word of
caution". The Lancet Respiratory Medicine Elsevier.
2020;8(4):e24. doi:10.1016/s2213-2600(20)30119-3. PMC 7118650.
PMID 32178774.
- "COVID-19 Treatment Guidelines". Available from:
https://www.covid19treatmentguidelines.nih.gov/. National
Institutes of Health. Retrieved 18 January 2021./
- Hsu, Jeremy. "Covid-19: What now for remdesivir?". BMJ.
2020;371:m4457. doi:10.1136/bmj.m4457. ISSN 1756-1833. PMID
33214186.
- Alwan NA, Burgess RA, Ashworth S, Beale R, Bhadelia N,
Bogaert D, et al. "Scientific consensus on the COVID-19
pandemic: we need to act now". Lancet. 2020;396(10260):e71–e72.
doi:10.1016/S0140-6736(20)32153-X. PMC 7557300. PMID 33069277.
- Meyerowitz-Katz G, Merone L. "A systematic review and
meta-analysis of published research data on COVID-19 infection
fatality rates". International Journal of Infectious Diseases.
2020;101: 138–148. doi:10.1016/j.ijid.2020.09.1464. PMC 7524446.
PMID 33007452.
- Zhang D, Hu M, Ji Q. "Financial markets under the global
pandemic of COVID-19". Finance Research Letters. 2020;36:101528.
Bibcode:2020CSFX....500043D. doi:10.1016/j.csfx.2020.100043. PMC
7402242. PMID 32837360.
- "Global Research and Innovation Forum on COVID-19: Virtual
Press Conference" . World Health Organization. Available from:
https://www.who.int/docs/default-source/coronaviruse/virtual-press-conference---2-july---update-on-covid-19-r-d.pdf
- Chen J, Jiang Q, Xia X, et al. Individual Variation of the
SARS-CoV-2 Receptor ACE2 Gene Expression and Regulation. Aging
Cell 2020;19(7):e13168.. doi: 10.1111/acel.13168.
- Heather N. Reich, Gavin Y. Oudit, Josef M. Penninger, James
W. Scholey and Andrew M. Herzenberg Decreased glomerular and
tubular expression of ACE2 in patients with type 2 diabetes and
kidney disease. 2008;74(12):1610-6. doi: 10.1038/ki.2008.497.
- Gavin Y.Oudit Michael A.Crackower Peter H.Backx Josef
M.Penninger The Role of ACE2 in Cardiovascular Physiology Trends
in Cardiovascular Medicine 2003;13(3):93-101.
https://doi.org/10.1016/S1050-1738(02)00233-5
- Oelkers WK. Effects of estrogens and progestogens on the
reninaldosterone system and blood pressure. Steroids
1996;61:166–71.
- Sampson AK, Moritz KM, Denton KM. Postnatal ontogeny of
angiotensin receptors and ACE2 in male and female rats. Gend Med
2012;9:21–32.
- Crackower MA, Sarao R, Oudit GY, et al.
Angiotensin-converting enzyme 2 is an essential regulator of
heart function. Nature 2002;417:822–8.
- Chaudhry F, Lavandero S, Xie X, et al. Manipulation of ACE2
expression in COVID-19. Open Heart 2020;7:e001424. doi:10.1136/
openhrt-2020-001424
- Zou X, Chen K, Zou J, et al. Single-Cell RNA-seq data
analysis on the receptor ACE2 expression reveals the potential
risk of different human organs vulnerable to 2019-nCoV
infection. Front Med 2020;14:185–92.
- Wölfel R, Corman VM, Guggemos W, et al. Virological
assessment of hospitalized patients with COVID-2019. Nature
2020;581:465–9.
- Bombardini T, Picano E. Angiotensin-Converting Enzyme 2 as
the Molecular Bridge Between Epidemiologic and Clinical Features
of COVID-19. Can J Cardiol. 2020;36(5):784.e1-784.e2. doi:
10.1016/j.cjca.2020.03.026. Epub 2020 Mar 29. PMID: 32299780;
PMCID: PMC7118531.
- Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin
converting enzyme 2 (ACE2) in SARS coronavirus-induced lung
injury. Nat Med 2005;11:875–9.
- Hsiao CH, Wu M-Z, Chen C-L, et al. Evolution of pulmonary
pathology in severe acute respiratory syndrome. J Formos Med
Assoc 2005;104:75–81.
- Gu J, Xie Z, Gao Z, et al. H5N1 infection of the respiratory
tract and beyond: a molecular pathology study. Lancet
2007;370:1137–45.
- Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, et al.
Angiotensin-Converting Enzyme 2 Metabolizes and Partially
Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects
in the Cardiovascular System. Hypertension. 2016;68(2):365-77.
doi: 10.1161/HYPERTENSIONAHA.115.06892. Epub 2016 May 23. PMID:
27217402.
- Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, et al.
Increased angiotensin-(1-7)-forming activity in failing human
heart ventricles: evidence for upregulation of the
angiotensin-converting enzyme Homologue ACE2. Circulation.
2003;108(14):1707-12. doi: 10.1161/01.CIR.0000094734.67990.99.
Epub 2003 Sep 22. PMID: 14504186.
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, et
al. A novel angiotensin-converting enzyme-related
carboxypeptidase (ACE2) converts angiotensin I to angiotensin
1-9. Circ Res. 2000;87(5):E1-9. doi: 10.1161/01.res.87.5.e1.
PMID: 10969042.
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, et al.
Hydrolysis of biological peptides by human
angiotensin-converting enzyme-related carboxypeptidase. J Biol
Chem. 2002;277(17):14838-43. doi: 10.1074/jbc.M200581200. Epub
2002 Jan 28. PMID: 11815627.
- Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, et al.
Angiotensin-Converting Enzyme 2 Metabolizes and Partially
Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects
in the Cardiovascular System. Hypertension. 2016;68(2):365-77.
doi: 10.1161/HYPERTENSIONAHA.115.06892. Epub 2016 May 23. PMID:
27217402.
- Kowalczuk S, Bröer A, Tietze N, Vanslambrouck JM, Rasko JE,
Bröer S. A protein complex in the brush-border membrane explains
a Hartnup disorder allele. FASEB J. 2008;22(8):2880-7. doi:
10.1096/fj.08-107300. Epub 2008 Apr 18. PMID: 18424768.
- Blume C, Jackson CL, Spalluto CM, Legebeke J, Nazlamova L,
et al. A novel ACE2 isoform is expressed in human respiratory
epithelia and is upregulated in response to interferons and RNA
respiratory virus infection. Nat Genet. 2021;53(2):205-214. doi:
10.1038/s41588-020-00759-x. Epub 2021 Jan 11. PMID: 33432184.
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell
RNA-seq data analysis on the receptor ACE2 expression reveals
the potential risk of different human organs vulnerable to
2019-nCoV infection. Front Med. 2020;14(2):185-192. doi:
10.1007/s11684-020-0754-0. Epub 2020 Mar 12. PMID: 32170560;
PMCID: PMC7088738.
- Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C.
The protein expression profile of ACE2 in human tissues. Mol
Syst Biol. 2020;16(7):e9610. doi: 10.15252/msb.20209610. PMID:
32715618; PMCID: PMC7383091.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor
H. Tissue distribution of ACE2 protein, the functional receptor
for SARS coronavirus. A first step in understanding SARS
pathogenesis. J Pathol. 2004;203(2):631-7. doi:
10.1002/path.1570. PMID: 15141377; PMCID: PMC7167720.
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner
AJ. A human homolog of angiotensin-converting enzyme. Cloning
and functional expression as a captopril-insensitive
carboxypeptidase. J Biol Chem. 2000;275(43):33238-43. doi:
10.1074/jbc.M002615200. PMID: 10924499.
- Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith
AI, Lew RA. The novel angiotensin-converting enzyme (ACE)
homolog, ACE2, is selectively expressed by adult Leydig cells of
the testis. Endocrinology. 2004;145(10):4703-11. doi:
10.1210/en.2004-0443. Epub 2004 Jul 1. PMID: 15231706.
- Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA
expression profiling of ACE 2, a novel homologue of angiotensin
converting enzyme. FEBS Lett. 2002;532(1-2):107-10. doi:
10.1016/s0014-5793(02)03640-2. PMID: 12459472.
- Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS et
al. Myocardial infarction increases ACE2 expression in rat and
humans. Eur Heart J. 2005;26(4):369-75; discussion 322-4. doi:
10.1093/eurheartj/ehi114. Epub 2005 Jan 25. PMID: 15671045.
- Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C.
The protein expression profile of ACE2 in human tissues. Mol
Syst Biol. 2020;16(7):e9610. doi: 10.15252/msb.20209610. PMID:
32715618; PMCID: PMC7383091.
- Takano H. Pulmonary surfactant itself must be a strong
defender against SARS-CoV-2. Med Hypotheses. 2020;144:110020.
doi: 10.1016/j.mehy.2020.110020. Epub 2020 Jun 20.
- Fandiño J, Toba L, Gonzalez-Nuñez M, Diz-Chaves Y,
Gonzalez-Matías L. & Mallo F, Liraglutide increases surfactant
proteins (SPA & SPB) and angiotensin-converting enzymes (ACE &
ACE2) expression in a rat model of acute lung injury by
bleomycin. Endocrine Abstracts 2016;41:GP99. DOI:
10.1530/endoabs.41.GP99.
- Mirastschijski U, Dembinski R and Maedler K. Lung Surfactant
for Pulmonary Barrier Restoration in Patients With COVID-19
Pneumonia. Front. Med. 2020;7:254. doi: 10.3389/fmed.2020.00254
- Severe acute respiratory syndrome (SARS). Available from:
https://www.malacards.org/card/severe_acute_respiratory_syndrome
- Respiratory Distress Syndrome in Premature Infants.
Available from:
https://www.malacards.org/card/respiratory_distress_syndrome_in_premature_infants
- Surfactant Metabolism Dysfunction, Pulmonary, 2. (SMDP2)
Available from:
https://www.malacards.org/card/surfactant_metabolism_dysfunction_pulmonary_2
- Pulmonary Fibrosis, Idiopathic. Available from:
https://www.malacards.org/card/pulmonary_fibrosis_idiopathic
- Idiopathic Interstitial Pneumonia. Available from:
https://www.malacards.org/card/idiopathic_interstitial_pneumonia
- Neonatal respiratory distress. Available from:
https://www.ncbi.nlm.nih.gov/clinvar
- ACE2 MalaCards. Available from:
https://www.malacards.org/search/results?query=ACE2
- Wright JR "Host Defense Functions of Pulmonary Surfactant".
Biology of the Neonate. 2004;85(4):326-32.
doi:10.1159/000078172. PMID 15211087. S2CID 25469141.
- Nkadi PO, Merritt, TA, Pillers De-Ann M. "An overview of
pulmonary surfactant in the neonate: Genetics, metabolism, and
the role of surfactant in health and disease". Molecular
Genetics and Metabolism. 2009;97(2):95–101.
doi:10.1016/j.ymgme.2009.01.015. ISSN 1096-7192. PMC 2880575.
PMID 19299177.
- Schurch S.; Lee M, Gehr P, Qanbar R, Schürch, S. "Pulmonary
surfactant: Surface properties and function of alveolar and
airway surfactant". Pure and Applied Chemistry. 1992; 64 (11):
209–20. doi:10.1351/pac199264111745. S2CID 97007574.
- Veldhuizen R, Nag K, Orgeig S, Possmayer F. "The role of
lipids in pulmonary surfactant". Biochimica et Biophysica Acta
(BBA) - Molecular Basis of Disease. 1998; 1408 (2–3): 90–108.
doi:10.1016/S0925-4439(98)00061-1. PMID 9813256.
- Wright , JR. "Host Defense Functions of Pulmonary
Surfactant". Biology of the Neonate. 2004; 85 (4): 326–32.
doi:10.1159/000078172. PMID 15211087. S2CID 25469141.
- Nkadi PO, Merritt TA, Pillers De-Ann M. "An overview of
pulmonary surfactant in the neonate: Genetics, metabolism, and
the role of surfactant in health and disease". Molecular
Genetics and Metabolism. 2009;97(2):95–101.
doi:10.1016/j.ymgme.2009.01.015. ISSN 1096-7192. PMC 2880575.
PMID 19299177
|
|
|
|

