| |
|
|
INTRODUCTION
Continuous supply of health institutions with sufficient amounts
of blood and blood products represents the main task of every
transfusion institution. Blood transfusion is one of the most common
interventions in medical practice since there is no effective
substitute for human blood. Having in mind that blood collection is
limited to healthy individuals, ensuring donor’s safety without
adverse reactions (ARs) is an essential factor that will encourage
them to donate blood and come back again in the near future. Blood
donation is voluntary, non-remunerated and anonymous. On the
territory of Serbia, all needs for blood and blood components are
met from one’s own sources [1]. In order to ensure sufficient
amounts of blood, it is necessary to take measures to retain the old
voluntary blood donors (BDs) and recruit new ones. With that in
mind, it’s important to implement a series of activities to motivate
the population and promote blood donation. Although blood donation
is considered a safe procedure with low risk rates, every potential
BD is thoroughly screened to ensure the safety of both the donor and
the recipient. Based on the Ordinance on donors of blood or blood
components (Službeni glasnik RS, No. 6/2019-132), any healthy person
aged from 18 to 65 years who fulfills the following criteria can be
a donor of blood or blood components:
a) good general condition and good venous access;
b) body weight of at least 50 kilograms;
c) adequate hemoglobin and hematocrit values (above 125 g/L and 0,38
L/L for a female; above 135 g/L and 0,40 L/L for a male);
d) body temperature less than 37 ℃, pulse 50-100 heartbeats per
minute;
e) blood pressure not higher than 180/100 mmHg and not lower than
100/60 mmHg.
After selection and examination of the BDs, blood for transfusion is
taken from the cubital vein into disposable bags of 450 mL so that
the amount of blood taken is up to 13% of the total volume of the
donor’s blood. Adverse reactions (ARs) in BDs are defined as any
adverse response associated with the collection of blood or blood
components and they occurred in about 1% to 5% of blood donations
[2]. They must be documented in the donor’s records, but also in the
records of the quality control system. Analysis of donor adverse
reaction reports will definitely help developing approaches to the
improvement of the overall safety of blood collection. According to
the recommendations of the Council of Europe and the Guide for the
preparation, use and quality assurance of blood components, 20th
edition, from 2020, a classification of complications related to
blood donation was performed [3].
ARs can be classified as:
a) Local complications: hematoma, arterial puncture, nerve injury or
compression, tendon injury, thrombophlebitis, local allergic
reaction, infection;
b) General complications: vasovagal reaction (VVR) (immediate or
delayed; at the venipuncture site or outside);
c) Other complications: generalized allergic reactions,
cardiovascular reactions (cardiac arrest, angina pectoris, cerebral
ischemia), accident or injury.
According to the severity, ARs can be divided into:
a) ARs which are not significant, classified as mild and moderate:
Hematoma: - local discomfort only during phlebotomy, minor pain or
functional impairment (mild)
local discomfort during phlebotomy, but also after the procedure,
when performing daily activities (moderate)
Arterial puncture: - without symptoms or local discomfort during
venipuncture, without hematoma (mild)
local discomfort that persists after blood collection is completed
(moderate)
Pain in the arm: - symptoms lasting less than 2 weeks (mild)
symptoms lasting more than 2 weeks but less than 1 year (moderate)
Vasovagal reactions: - subjective symptoms only (mild)
objective symptoms (moderate)
b) Adverse reactions associated with blood collection that could
lead to incapacitation of the donor and result in hospitalization
and morbidity are defined as severe reactions such as delayed
syncope, cardiac arrest, collapse with convulsions, cerebral
ischemia.
AIM
The aim of this study was to determine the frequency and severity of
ARs that occurred among BDs on the territory of Vojvodina by
analyzing the age and profile of donors in whom they were recognized
but also to indicate possible prevention of ARs.
MATERIAL AND METHODS
In a retrospective study, the records of ARs among whole BDs at
the Blood Transfusion Institute Vojvodina, from January 1, 2017,
until December 31, 202 were analyzed. Depending on the number of
blood donations, donors are categorized into first-time and multiple
donors. Demographic data of the donors related to age, gender,
number of donations and place of donation were obtained from the
Institute's information system. Depending on the time of occurrence,
a classification and analysis of ARs were performed on those that
occurred before the beginning of the blood donation procedure,
during the procedure and after the procedure is completed. According
to the type of occurrence, ARs were divided into local and systemic
reactions, while, according to the severity, they were classified
and analyzed into mild, moderate, and severe.
The data were analyzed and processed using the methods of
descriptive statistics in the Minitab 16 software program. The
following descriptive statistical parameters were used: arithmetic
mean, standard deviation and median. ANOVA was used to assess the
statistical significance of the obtained results with a significance
level of less than 0.05. The findings are presented in tabular and
graphical form.
RESULTS
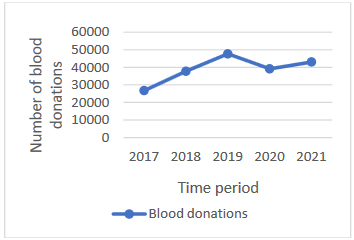
During the study period, 194,425 donations were collected and
analyzed, in which 108,014 voluntary donors of whole blood
participated (Graph 1). Among the blood donors, there were 83.678
(77.47%) men and 24,336 (22.53%) women (the male/female ratio was
3:1). There were 99,524 (92.14%) multiple donors and 8,490 (7.86%)
first-time donors.
Graph 1. Number of blood donations in the period
from 2017 to 2021
Based on the total number of donations, adverse reactions
occurred during 2722 procedures (1.4%). The number of incidence was
14 in every 1000 donations. BDs who experienced ARs were 28,0 ± 8,3
years old, 1881 (69.1%) were male and 841 (30.9%) were female.
People who donated blood for the first time were at a higher risk of
experiencing an adverse reaction, which happened to 1908 people
(70.1%), while multiple donors were less represented, merely 814 of
them (29.9%). Donors from urban regions were more represented, 2,349
of them (86.3%), while there were 373 (13.7%) from rural regions.
The chi-square test was used to analyze the occurrence of adverse
reactions in men 1811/83678 (2.16%) in regards to women 841/24336
(3.45%) and a highly significant statistical difference was
determined (p<0.001). Also, the chi-square test was used to analyze
the occurrence of adverse reactions in first-time BDs 1908/8490
(22.5%) compared to 814/99524 (0.82%) multiple ones and a
statistically significant difference was also determined (p<0.001).
According to the time of occurrence of the adverse reaction, it was
observed that the most ARs occurred during blood donation procedure,
1717 (63.1%). After the blood donation procedure was completed, 893
(32.8%) ARs were identified, while112 ARs (4.1%) were identified
before the procedure.
In relation to localization, systemic reactions predominated in 2619
(96.2%) donors, while local reactions occurred in 103 (3.8%). BDs
who were younger than 30 years and weighed less than 60 kg had
vasovagal reactions, nausea and syncope more often (p<0.005). The
occurrence of local and systemic ARs in BDs in relation to their
donor status is shown in Table 1.
Table 1. Types of adverse reactions during whole
blood donation procedure
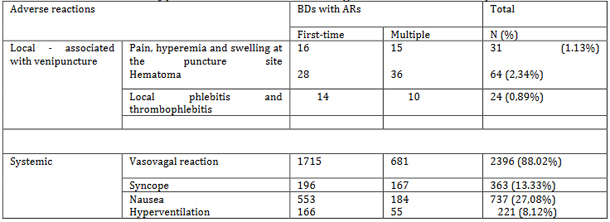
In relation to the severity of the ARs, 64 (2.34%) BDs had mild
reactions in the form of hematoma, and 2396 (88.03%) of them
experienced weakness and fainting. Moderate ARs in the form of
nausea and sweating occurred in 737 (27.08%) donors. Severe ARs in
the form of collapse with convulsions were experienced by 12 (0.44%)
donors. Adverse reactions were mostly mild and moderate (p<0.05).
DISCUSSION
Caring for donors primarily means protecting their own health. In
addition, any inconvenience associated with blood donation procedure
may result in refusing BDs to come again. Conversely, the safe and
pleasant experience that BDs have when donating blood can encourage
them to come again and be recruited and motivated to become regular
blood donors, which consequently leads to a satisfactory supply of
blood units. France et al. indicate that donors give up further
blood donation after experiencing an adverse reaction [4]. It is
very important to analyze ARs related to blood donation, to consider
what affects their occurrence so that preventive measures and
adequate care can be taken. Several authors dealt with the
consideration of the factors that influence the emergence of ARs and
the question of which the weakest links in the chain of work
processes with BDs are, in order, first of all, to correct them and
thereby increase the satisfaction of DDK [5,6]. In case of
occurrence of ARs, the most important thing is to provide adequate
professional help in a timely manner, determine the cause of the
occurrence, and, after analysis, implement corrective and preventive
measures. Each adverse reaction with all the measures taken is
recorded in the donor's file, while it is a legal obligation to
report severe ARs to the hemovigilance system at the national level.
The frequency of ARs certainly depends on several factors, from the
preparation of the BD for the blood donation procedure, his/her
general condition and hydration, venipuncture, conversation with
him/her during the procedure, as well as the provision of
post-donation information about behavior after donating blood. In
our population of whole blood donors, the incidence of total ARs is
1.4%, while according to published data it ranges from 0.03% to 6%
[7,8]. The difference in the mentioned data may be due to the wide
range of ARs that were analyzed, as well as the size of the
population that was the subject of the research.
On the other hand, most reported ARs are systemic, but even here
there are significant differences. While in our study systemic
adverse reactions in relation to the total number of donations
occurs with an incidence of about 1%, in Greece the frequency is
0.88%, and in Japan 6% [9,10]. Systemic reactions are influenced by
many factors, in which the most important are age, gender, stress,
fluid intake, proper diet and adequate sleep before donating blood.
Vasovagal reaction is the most common type of AR in our population
with a share of about 88% in relation to all other reactions and
1,23% in relation to the total number of donations. The incidence of
VVR varies among different populations. Agnihotri et al. published
the data that among whole blood donors, 1.6% have VVR, and these
are, above all, younger women donating blood for the first time
[11]. Dogra et al. came to a similar conclusion, although the
incidence was much lower and amounted to 0.365% [12]. VVR represents
the reaction of the neurovegetative system to stress, which can also
have a cause in acute blood loss. Although it has a low incidence,
it can have a long-term negative impact on the return rate of BDs
and is often the main reason donors refrain from coming back. The
most vulnerable group of BDs for the occurrence of ARs are high
school students weighing up to 55 kg who are donating blood for the
first time. Since VVR are the most represented, a seasonal variation
in their manifestation was also observed because it correlated with
periods when there was a higher representation of high school
students in organized blood donation actions. Sultan et al. and
Tondon et al. indicated a positive correlation between the increase
in the age categories of BDs with a decrease in the risk of VVR
[10,13]. The reason for this correlation lies in the fact that
younger people have a greater sensitivity of the carotid-aortic
baroreceptor, which can be the cause of VVR if the receptor is
stimulated during or after the donation process. As the age of DDK
increases, baroreceptor sensitivity decreases, which explains the
decrease in VVR incidence in older age groups. Many studies have
indicated that female gender is more associated with the occurrence
of ARs, primarily due to the difference in blood pressure. It has
been proven that there are gender differences in the
renin-angiotensin system and the effects of the bound angiotensin II
type 2 receptor on renal vascular resistance, whereby renal
sympathetic nervous activity affects the value of blood pressure
[14]. The data of our study indicate that in relation to the
majority of ARs, the predominant clinical form are mild and moderate
adverse reactions, while severe forms occur very rarely. These
findings are consistent with data from many other studies, which
indicate the fact that blood donation is a safe procedure, mostly
without complications [15-17].
It is important to implement all mechanisms that could prevent ARs,
especially when it comes to BDs who are donating blood for the first
time. These procedures include: the shortest possible waiting time
for BDs, from arrival to the venipuncture itself, in a pleasant
environment, good psychological preparation for BDs, pre-donation
hydration, performing muscle tension exercises, hiring experienced
staff and good puncturers [4,18-20]. Communication with blood donors
is extremely important and it is considered that no other prevention
measure can replace it.
CONCLUSION
Continuous attention and monitoring of donors during the entire
blood donation procedure contribute to a low incidence of adverse
reactions. Education of the medical personnel to identify risk
factors contributes to the prevention of adverse reactions.
Prevention measures of adverse reactions, as well as their quick
treatment, are important because of the preservation of donor's
health and the negative effects they have on the motivation of the
donors and their return.
REFERENCES
- Ministarstvo zdravlja Republike Srbije. Zakon o
transfuziološkoj delatnosti. Sl. glasnik RS br. 40/2017.
Beograd; 2017.
- Taheri Soodejani M, Haghdoost AA, Okhovati M, Zolala F,
Baneshi MR, Sedaghat A, et al. Incidence of adverse reaction in
blood donation: a systematic review. Am J Blood Res.
2020;10(5):145-150. PMID: 33224558; PMCID: PMC7675132.
- European Committee on Blood Transfusion. Guide to the
preparation, use and quality assurance of blood components. 20th
ed. Strasbourg: European Directorate for the Quality of
Medicines and Health Care; 2020.
- France CR, France JL, Wissel ME, Ditto B, Dickert T, Himawan
LK. Donor anxiety, needle pain, and syncopal reactions combine
to determine retention: a path analysis of two-year donor return
data. Transfusion. 2013;53(9):1992-2000. doi: 10.1111/trf.12069.
PMID: 23305267; PMCID: PMC3626759.
- Wang HH, Chen PM, Lin CL, Jau RC, Hsiao SM, Ko JL. Joint
effects of risk factors on adverse events associated with adult
blood donations. Medicine 2019;98(44):e17758. doi:
10.1097/MD.0000000000017758. PMID: 31689834; PMCID: PMC6946510.
- Wiersum-Osselton JC, Marijt-van der Kreek T, Brand A,
Veldhuizen I, van der Bom JG, de Kort W. Risk factors for
complications in donors at first and repeat whole blood
donation: a cohort study with assessment of the impact on donor
return. Blood Transfus. 2014; 12 (Suppl 1): s28-36. doi:
10.2450/2013.0262-12. PMID: 23867173; PMCID: PMC3934284.
- Prakash S, Das PK, Mishra D, Ray GK, Routray S, Naik A, et
al. Incidence and risk predictors analysis of adverse donor
reactions in whole blood donation. Transfusion Clinique et
Biologique 2020;27(4):207-12.
- Rahman A, Bhuiyan MZR, Dey BP, Rassel M. Incidence of
immediate adverse reaction of blood donation. Bangladesh Med J.
2016;45(2):75-8.
- Zervou EK, Ziciadis K, Karabini F, Xanthi E, Chrisostomou E,
Tzolou A. Vasovagal reactions in blood donors during or
immediately after blood donation. Transfus Med.
2005;15(5):389-94. doi: 10.1111/j.1365-3148.2005.00600.x. PMID:
16202053.
- Sultan S, Baig MA, Irfan SM, Ahmed SI, Hasan SF. Adverse
Reactions in Allogeneic Blood Donors: A Tertiary Care Experience
from a Developing Country. Oman Med J. 2016;31(2):124-8. doi:
10.5001/omj.2016.24. PMID: 27168923; PMCID: PMC4861393.
- Agnihotri N, Marwaha N, Sharma RR. Analysis of adverse
events and predisposing factors in voluntary and replacement
whole blood donors: A study from north India. Asian J Transfus
Sci. 2012;6(2):155-60. doi: 10.4103/0973-6247.98922. PMID:
22988381; PMCID: PMC3439755.
- Dogra A, Sidhu M, Dogra M, Raina TR. Study of adverse whole
blood donor reactions in normal healthy blood donors: Experience
of tertiary health care centre in Jammu region. Indian J Hematol
Blood Transfus. 2015;31:142–5.
- Tondon R, Pandey P, Chaudhary R. Vasovagal reactions in 'at
risk' donors: A univariate analysis of effect of age and weight
on the grade of donor reactions. Transfus Apher Sci.
2008;39(2):95–9.
- Morand C, Coudurier N, Rolland C, Thoret S, Legrand D,
Tiberghien P, et al. Prevention of syncopal-type reactions after
whole blood donation: a cluster-randomized trial assessing
hydration and muscle tension exercise. Transfusion
2016;56(10):2412–21.
- Philip J, Sarkar RS, Jain N. A single-centre study of
vasovagal reaction in blood donors: Influence of age, sex,
donation status, weight, total blood volume and volume of blood
collected. Asian J Transfus Sci. 2014;8(1):43-6. doi:
10.4103/0973-6247.126690. PMID: 24678174; PMCID: PMC3943146.
- Locks MOH, Salum NC, Barros BS, Matos E, Anders JC,
Schneider DG. Profile of blood donors who presented adverse
reactions to the donation. Rev Bras Enferm. 2019;72(1):81-7. doi:
10.1590/0034-7167-2018-0305. PMID: 30916271.
- Hasan I, Arshad A, Rahim NA, Soo PY. Vasovagal reaction
among whole blood donors in Hospital Pulau Pinang. A
statistical-epidemiological study. Asian J Transfus Sci.
2020;14(1):28-32. doi: 10.4103/ajts.AJTS_111_17. PMID: 33162702;
PMCID: PMC7607985.
- Newman B, H: Management of Young Blood Donors. Transfus Med
Hemother. 2014;41:284-95. doi: 10.1159/000364849.
- Thijsen A, Gemelli CN, Davison TE, O’Donovan J, Bell B,
Masser B. Does using applied muscle tension at strategic time
points during donation reduce phlebotomist-and donor-reported
vasovagal reaction rates? A three-armed randomized controlled
trial. Transfusion 2018;58:2352–9.
- France CR, France JL, Conatser R, Lux P, McCullough,
Erickson Y. Predonation fears identify young donors at risk for
vasovagal reactions. Transfusion 2019;59:2870–5.
|
|
|
|


