| |
|
|
INTRODUCTION
High blood pressure (BP) is the leading correctible risk factor
for chronic diseases in the world [1]. High BP is not only an
important risk factor for chronic kidney disease (CKD) [2], but also
an important comorbidity that occurs with a prevalence of 86% in the
population of patients with CKD not receiving dialysis [3]. The
combination of CKD and hypertension leads to a high risk of
cardiovascular disease (CVD), which is the most common cause of
morbidity and mortality in patients with CKD [4]. Several clinical
studies and meta-analyses have shown that aggressive treatment of
hypertension in patients with and without CKD reduces the risk of
CVD, as well as all-cause mortality, although the protective effects
of BP reduction on renal function remain controversial [5,6]. For
these reasons, several different guides/guidelines for the treatment
of hypertension in CKD have been published so far, the last few of
which are listed in Table 1.
Table 1. Comparison of several recent hypertension
guidelines
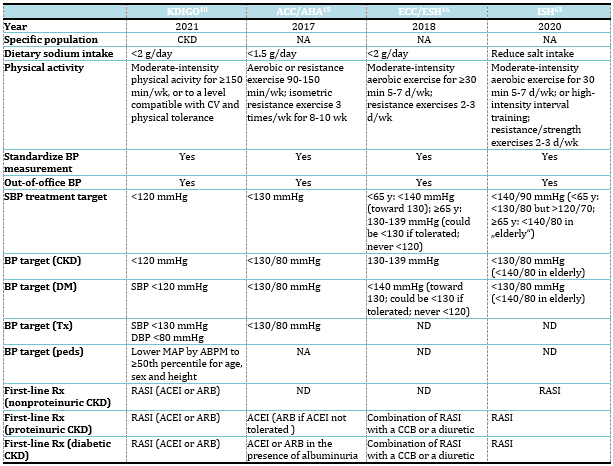
Abbreviations: ABPM, ambulatory blood pressure
monitoring; ACEI, angiotensin-converting enzyme inhibitor; ARB,
angiotensin II receptor blocker; BP, blood pressure; CCB, calcium
channel blocker; CKD, chronic kidney disease; CV, cardiovascular;
DM, diabetes mellitus; MAP, mean arterial pressure; NA, not
applicable; ND, not discussed; peds, pediatric; RASI,
renin-angiotensin system inhibitors; Rx, prescription; SBP, systolic
blood pressure; Tx, transplant;
The original KDIGO (Kidney Disease: Improving Global Outcomes)
clinical practice guideline for the management of blood pressure in
the population of CKD patients not receiving dialysis was published
in 2012. [7]. Since then, several articles have been published
reporting on the primary results and important secondary analyses of
large, randomized trials of hypertension treatment in various
populations, including patients with CKD. Intensive lowering of
systolic blood pressure (SBP) to a target of 120 mmHg in SPRINT
(Systolic Blood Pressure Intervention Trial) reduced the risk for
CVD and all-cause mortality to a similar extent in patients with and
without CKD [5].Secondary combined analyzes of SPRINT and ACCORD-BP
(Action to Control Cardiovascular Risk in Diabetes-Blood Pressure)
trials showed a similar reduction in the primary composite outcome
of CVD and all-cause mortality for the SPRINT study and the standard
glycemic control arm of the ACCORD-BP trial [8]. In the VA NEPHRON-D
study (Veterans Affairs Nephropathy in Diabetes), combination
therapy with angiotensin converting enzyme inhibitors (ACEIs) and
angiotensin receptor blockers (ARBs) increased the risk of acute
kidney injury (AKI) and hyperkalaemia, and showed no benefit for
renal or cardiovascular outcomes. [9]. In 2017, KDIGO undertook a
multi-year process of updating its original guideline, and the
results of these and many other studies are included in the updated
guideline published in 2021. [10].
The 2021 revision of the KDIGO guideline also applies only to the
population of patients with CKD not receiving dialysis and it covers
topics contained in the original guideline, such as optimal blood
pressure targets, lifestyle interventions, choice of
antihypertensive drugs and specific management in kidney transplant
recipients and children (Table 2). Some aspects of general and
cardiovascular health, such as lipid control and smoking, are
excluded. A Work Group of researchers and clinicians working on the
revision of the original guideline identified 2 major areas that
warrant particular attention due to the emergence of new evidence:
BP measurement and BP target in patients with CKD. These 2 problems
are closely related, because the target SBP <120 mmHg depends on the
proper adherence to standardized preparation and measurement
protocols by patients and clinicians. On the other hand, the main
objections are also aimed to these 2 focuses: the observed
impracticality of standardized BP measurement in clinical practice
and the difficulty in achieving new SBP targets [10].
Table 2. Key recommendations from KDIGO 2021
Clinical Practice Guideline for BP Management in CKD.
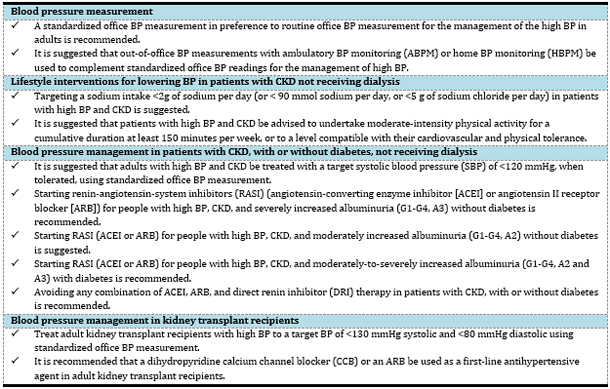
BLOOD PRESSURE MEASUREMENT
Sphygmomanometry is the first practical method that Riva Rocci
introduced in 1896 for estimation of SBP [11]. Diastolic blood
pressure (DBP) readings became feasible in 1905, when Korotkov
described his auscultatory method of measurement [20]. It was soon
noticed that BP varies dramatically from one reading to another, so
attention was focused on standardizing BP measurement methods to
avoid errors in estimation. However, despite all the issued
guidelines, recommendations and specific approaches to improve the
accuracy of measuring BP, a recent meta-analysis documented that the
average SBP in routine clinical practice is almost 15 mmHg higher
than in research studies [13].
Chapter 1 is a new addition to the original KDIGO BP guideline that
highlights the importance of accurate BP measurement in adults.
Standardized office BP refers to measurements obtained in accordance
with recommended preparations and measurement techniques, regardless
of the type of equipment used, as opposed to routine office BP
measurements performed without these preparations Standardized BP
measurement is an integral part of BP target and the BP target
guideline cannot rely on routine BP measurements, because large
randomized trials that examined target BP, including SPRINT and
ACCORD, have consistently used standardized BP measurements [10].
Furthermore, strong evidence shows that routine office BP and
standardized office BP measurements do not give the same values, and
the relationships between these 2 techniques are highly variable, so
it is not possible to use some correction factor to convert routine
values to standardized BP values [14]. The KDIGO recommendations for
measuring standardized BP are in line with other recent guidelines
[15-18], but what makes a critical distinction is the insistence on
widespread adoption of standardized BP measurement in patients with
CKD, because it allows the use of lower target SBP with proven
efficacy in clinical trials.
Key elements for successful BP measurement in the office include
proper patient preparation, use of a validated measuring device,
correct techniques, and average BP values from at least 2
measurements (Table 3). Patients should be instructed to empty their
bladder and avoid smoking, caffeine, and physical activity for at
least 30 minutes before measuring their BP. They should be seated
comfortably with their back supported and feet on the ground > 5
minutes before the readings. The patient and the observer should
refrain from talking during the rest period and during BP
measurement. The patient's arm should be supported, and all clothing
covering the location of cuff placement should be removed. Cuff size
should correspond to the circumference of the patient's arm, and the
cuff should be placed at heart level (the midpoint of the sternum).
The guidelines recommend using an average 2 or more readings
obtained on 2 or more occasions to estimate the individual's level
of BP. Patients should be informed of their BP values [10,15-18].
Table 3. Checklist for standardized measurement of
blood pressure in the office

A variety of BP measurement devices can be used for standardized
office BP measurement, because the emphasis of standardization is on
adequate preparation of patients for BP measurement, and not on the
type of equipment [10]. However, there are several reasons why
oscillometric devices are now considered a clinical standard for BP
measurement [15, 18]: environmental concerns about mercury toxicity,
the need for frequent calibration with aneroid sphygmomanometers,
errors due to auscultation and inappropriately rapid deflation of
the cuff, and greater convenience and cost savings associated with
use of oscillometric devices [18]. Oscillometric devices can be used
to measure BP in patients with atrial fibrillation [10]. Given that
large randomized studies have not found significant differences
between standardized BP values measured using oscillometric and
manual devices, manual BP devices are also considered acceptable
when oscillometric devices are unavailable [19, 20]. Automated
office BP devices may be the preferred method for standardized
office BP measurement. They may increase the likelihood of adherence
to proper preparation because they can be programmed to include a
rest period, and they can also take multiple BP measurements and
provide an average. Automated devices can measure BP either with or
without a health worker in the room. The results of the SPRINT trial
indicate attended or unattended automated office BP measurements
result in similar BP values when the recommendations for accurate BP
measurement are followed [21,22].
Out-of-office BP measurement techniques include home BP monitoring (HBPM)
and 24-hour ambulatory BP monitoring (ABPM). In patients not taking
BP-lowering medications, the following 4 groups can be categorized
based on in-office and out-of-office BP measurements (Figure 1):
normotension, white coat hypertension, sustained hypertension, and
masked hypertension. In those taking BP lowering medications, 4
similar groups can be categorized: white coat effect, sustained
controlled hypertension, sustained uncontrolled hypertension, and
masked uncontrolled hypertension. [10].Approximately 30% of patients
have discordant BP values in-office and out-of-office [23].Masked
uncontrolled hypertension is more common in people with CKD compared
to people without CKD [24]. Masked hypertension is associated with
an increased risk of CVD and renal failure. In contrast, white coat
hypertension is associated with a lower risk of adverse events than
masked and sustained hypertension, but patients with untreated white
coat hypertension have a higher risk of adverse events than patients
with controlled office and out-of-office BP [25]. The high
prevalence of white coat hypertension and masked hypertension, as
well as the increased risk of adverse outcomes identified in
observational studies, have resulted in the recommendation that ABPM
and HBPM be used to complement standardized office BP for the
management of high BP [10,15-17].
Figure 1. BP patterns based on out-of-office BP
measurements in addition to standardized office BP measurements.
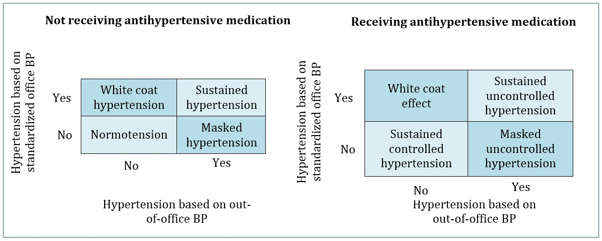
The KDIGO BP guideline recommends that ABPM be used initially to
supplement standardized office BP measurement, while HBPM is further
used for ongoing BP management. In areas where ABPM service is not
available, HBPM may be used instead of ABPM as the initial
procedure. Out-of-office BP measurement additionally burdens
patients and clinic staff. For example, ABPM requires patients to
wear a monitor for 24 hours, with the obligation to visit the clinic
on 2 consecutive days for placement and removal of the monitor. On
the other hand, HBPM is a more accessible method and can be
particularly important for the management of BP when a visit to the
clinic is impossible or difficult for some reason. As with all BP
measurements, out-of-office readings should be obtained using the
standardized technique and a validated upper arm
device.Notwithstanding the recommendations made, the KDIGO work
group recognized the lack of randomized controlled trials comparing
the effect of ABPM/HBPM to office-based BP management on
cardiovascular or kidney disease outcomes, and therefore supports
further research in this area [10].
LIFESTYLE INTERVENTIONS
According to the KDIGO guideline, the suggested sodium intake
should be<2 g of sodium per day (or <90 mmol of sodium per day, or
<5 g of sodium chloride per day) in patients with high BP and CKD
[10]. Interventional studies in the general population have shown a
gradual benefit in reducing of both BP and CVD risk with reduced
dietary sodium intake [26]. Although the majority of the world's
population consumes more than 2 g of sodium per day, even modest
reductions in sodium intake that do not reach this goal are
associated with BP and CVD benefits in the general population.
However, there are no large and long-term randomized controlled
trials evaluating the effects of dietary sodium restriction on
clinical outcomes in CKD population.A recent meta-analysis that
included only studies with CKD patients found that salt reduction in
patients with CKD significantly reduced BP, and if such an effect
were maintained in the long term it would result in a clinically
significant reduction in CKD progression and CV events [28].
Finally, ACEI and ARB medications are commonly used in CKD
population, and their kidney and cardiovascular benefits may be
improved if accompanied by a low-sodium diet [29].
Considering that data on specific targets of sodium intake in CKD
population with high BP are not firmly established, the KDIGO work
group has adopted the recommended target for dietary sodium intake
in the general population from the World Health Organization [30],
which is in line with the recommendations of several recently
published guides to hypertension [16, 17], but also consistent with
KDIGO 2020 Guideline for Diabetes Management in CKD [31]. The WG
also noted that there are circumstances in which recommendations
from the general population cannot be applied to CKD population. The
warnings relate to patients with CKD and salt-wasting nephropathy,
for whom reduction in sodium intake may be inappropriate. The second
warning relates to the dietary approach to the treatment of
hypertension, taking into account that diets employed to lower BP
are usually rich in potassium, and salt substitutes also contain
potassium as the primary cation. These approaches may increase the
risk of hyperkalemia, especially in advanced CKD [10].
As part of lifestyle changes, patients with high BP and CKD are
advised to undertake moderate-intensity physical activity for a
cumulative duration of at least 150 minutes per week, or to a level
compatible with their cardiovascular and physical tolerance. Studies
in the general population have clearly demonstrated the beneficial
effects of physical activity on BP-lowering, physical fitness and
strength, weight loss and reducing the risk of dysglycemia and
diabetes [10]. In the CKD population, the evidence is much more
limited, but it also suggests that physical activity reduces BP and
body weight and improves quality of life [32,33]. Observational data
have shown a dose-response relationship between higher levels of
physical activity and lower risk of mortality in patients with CKD
[34]. On the other hand, the KDIGO work group recognizes a higher
prevalence of comorbidities and frailty in the CKD population that
might limit the level of physical activity by CKD patients and
increase the risk of adverse events. Therefore, the degree of
physical activity should be individualized in accordance with the
patient's cognitive and physical conditions , which may change over
time. Significant health benefits can be gained even if the level of
physical activity falls below the proposed targets [10].
BLOOD PRESSURE TARGETS
In adults with high BP and CKD, the KDIGO guideline suggests a
target SBP of <120 mmHg when tolerated, provided that a standardized
office BP measurement is used. This recommendation pertains to
patients with diabetes and without diabetes, and does not apply to
patients with a kidney transplant or to those receiving dialysis
[10].
For most patients with CKD, particularly those who are older, with
low levels of albuminuria or are in the earlier stages of CKD, the
risks for CVD and CV mortality are much higher than those for kidney
failure [35]. Therefore, this KDIGO recommendation relies heavily on
the results of a high-quality and randomized SPRINT, that showed
beneficial effects on CV and mortality outcomes in a study cohort of
hypertensive subjects randomized to a target SBP <120 mmHg versus
140 mmHg [5].In this study, 90% of participants were receiving
antihypertensive therapy at baseline, and beneficial effects were
demonstrated in the group of patients with CKD [36], in the elderly
[37] and in those with prediabetes [38]. Two meta-analyses also
reported a risk reduction for CV events with intensive BP lowering
in the CKD population, regardless of whether the reduction was equal
to [39] or lower than in the general population [40].
The effects of intensive BP lowering on CKD progression toward
kidney failure are less certain. There is a common perception that
BP lowering is renoprotective, but only secondary analyses of some
earlier randomized trials have shown that more intensive BP lowering
reduces the rate of CKD progression among patients with greater
baseline proteinuria [41,42]. However, the results of the two most
frequently cited recent randomized trials, SPRINT and ACCORD,
indicate that intense BP lowering leads to a small but consistent
reduction in estimated glomerular filtration rate (eGFR) shortly
after initiation, compared to less intensive controls (may be
explained by hemodynamic effects), while the effects of intensive BP
lowering on eGFR in the long term remain uncertain [36,43].
The original KDIGO 2012 BP guideline recommended a more intensive BP
lowering for patients with albuminuria than those without [7]. With
the adoption of an SBP target bellow 120 mmHg for all CKD patients
in the revised guideline, separate targets for patients with and
without albuminuria were no longer considered necessary. The KDIGO
work group considered that the cardiovascular and survival benefits
of intensive SBP control outweighed the observed increases in the
risks for hyperkalaemia, hypokalaemia and acute renal injury
[36].However, evidence supporting the SBP target <120 mmHg is less
certain in some subpopulations, including patients with diabetes,
advanced CKD (G4 and G5), significant proteinuria (> 1 g/day),
baseline SBP 120-129 mmHg, in younger than 50 years or very old
(age> 90 years), as well as those with "white coat" or severe
hypertension [10], table 4. For example, the ACCORD trial studied
exclusively patients with diabetes and randomized them to the same
SBP targets as in SPRINT (<120mmHg, vs<140mmHg), but excluded those
with a serum creatinine levels >132.6 umol/L and those with
proteinuria >1g/day. Intensive BP control resulted in a lower risk
for stroke, but without a statistically significant reduction in
overall CV events. The analyses of ACCORD suggest a CV benefit of
the lower BP target in the group with standard glucose control, but
not in the group with intensive glucose control [8,43,44].However,
for a similar SBP lowering, there was a greater risk of eGFR decline
in patients with diabetes in ACCORD-BP than in patients without
diabetes in SPRINT [45]. Therefore, the KDOQI (Kidney Disease
Outcomes Quality Initiative) work group commented that the
risk-benefit ratio of lower SBP target in patients with CKD and
diabetes requires further research in randomized controlled trials,
and currently considers an SBP target of <130 mmHg to be a more
reasonable target in this subpopulation [46].
Table 4. Certainty of evidence supporting an SBP
target of <120 mmHg for patients with CKD
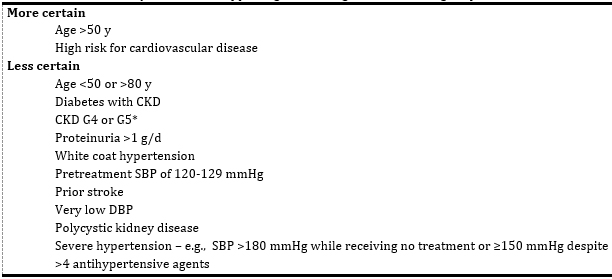
Abbreviations: CKD, chronic kidney disease; DBP, diastolic blood
pressure; SBP, systolic blood pressure;
*CKD G4-G5 indicates estimated glomerular filtration rate <30
ml/min/1.73m2.
Uncertainty about benefits and risks of intensive BP lowering in
different subpopulations does not mean that intensive BP lowering is
not warranted, but suggests that the potential adverse effects
should be taken into consideration when deciding on the BP target
for individual patients. Inconsistency in recommendations for
treatment target SBP may contribute to physician confusion: ACC/AHA
(American College of Cardiology/American Heart Association) BP
guideline offers a target of<130/80 mmHg for patients with CKD,
which is more aggressive than that recommended by the European
Society of Cardiology/European Society of Hypertension (ESC/ESH; SBP
target 130-139 mmHg), and different from that recommended by the
National Institute of Health and Care Excellence (NICE; SBP target
120-139 mmHg) [15,16,47]. ESC also published a 2021 Clinical
Guideline on Cardiovascular Disease Prevention in Clinical Practice
that recommend office BP targets for people with CKD<140–130 mmHg
SBP (lower SBP is acceptable if tolerated) and <80 mmHg DBP [48]. In
practice, it should be borne in mind that it would be potentially
hazardous to apply the recommended SBP target of <120 mmHg to BP
measurements obtained in a non-standardized manner. It is also
reasonable to use less intensive therapy for BP lowering in patients
with very limited life expectancy or symptomatic orthostatic
hypotension [10].
CHOICE OF ANTIHYPERTENSIVE DRUGS
Recommendations for the choice of antihypertensive therapy in CKD
are based on evidence that renin-angiotensin system inhibitors (RASI),
ACEI and ARB, reduce both CV events rates and kidney end points
among patients with CKD. The strength of the evidence varies from
strong in the CKD subpopulation with low eGFR and severely increased
albuminuria to weak or absent in the subpopulation with normal eGFR
without albuminuria. Many patients with CKD will need a combination
of 2 or more antihypertensive drugs, but there are no randomized
controlled trials comparing different combination therapies in CKD.
Therefore, any algorithm for antihypertensive treatment in CKD is
based on expert opinion, pathophysiological or pharmacodynamic
considerations, or extrapolation from findings in the general
population [10].
In patients with high BP, CKD(G1-G4) and severely increased
albuminuria (A3) without diabetes, it is recommended to start RASI
therapy (ACEI or ARB) [10]. Evidence supporting this view is based
on the results of several placebo-controlled randomized trials,
which confirmed the effects of this therapy on reducing the risks
for both adverse renal outcomes and CV events [49-51].
In patients with high BP, CKD (G1-G4) and moderately increased
albuminuria (A2) without diabetes, it is recommended to start RASI
therapy (ACEI or ARB) [10]. This is a weak recommendation, because
there is no high-quality evidence from randomized controlled trials
evaluating kidney outcomes in this subpopulation. The recommendation
relies heavily on the results of the HOPE (Heart Outcomes and
Prevention Evaluation) trial, which showed a CV benefit of ramipril
compared to placebo, independent of BP, in patients with moderately
increased albuminuria [52].
In patients with high BP, CKD (G1-G4) and moderate to severe
albuminuria (A2 and A3) with diabetes, it is recommended to start
RASI therapy (ACEI or ARB) [10]. Strong evidence from IDNT (Irbesartan
Diabetic Nephropathy Trial) and RENAAL (Reduction of Endpoints in
NIDDM with the Angiotensin II Antagonist Losartan) indicates that
RASI, compared with placebo or calcium channel blockers (CCBs),
reduces risk for kidney events in diabetics with severely increased
albuminuria [53,54]. MICROHOPE (Microalbuminuria, Cardiovascular,
and Renal Outcomes Substudy of Heart Outcomes Prevention Evaluation)
found a reduction in CV risk in patients with diabetes and moderate
albuminuria who were randomized to ramipril [55,56]. Meta-analysis
by the KDIGO ERT (Evidence Review Team) showed that ACEIs compared
with placebo had no effect on all-cause mortality but significantly
reduced the risk for doubling of serum creatinine and progression of
albuminuria from category A2 to A3 [10].
The KDIGO guideline highlights several practical points to pay
attention to. The first point suggests that RASI (ACEI or ARB) would
be a reasonable choice of therapy for people with high BP, CKD, and
no albuminuria, with or without diabetes.Based on some research
[57], the KDOQI work group believes that a diuretic or CCB would be
equally reasonable choice as a first-line treatment for high BP in
patients with CKD and without diabetes and no albuminuria [46],
which is also recommended by the ACC/AHA guideline [15]. The need to
use RASI (ACEI or ARB) in the highest approved dose that is
tolerated is further emphasized, because the described benefits were
achieved in trials using these doses. Changes in BP, serum
creatinine, and serum potassium should be checked within 2-4 weeks
of initiation or increase in the dose of a RASI, depending on the
current eGFR and serum potassium. Hyperkalaemia associated with use
of RASI can often be managed by measures to reduce serum potassium
levels, rather than decreasing the dose or discontinuing RASI. ACEI
or ARB therapy should be continued unless serum creatinine rises by
more than 30% within 4 weeks of starting treatment or increasing the
dose. Dose reduction or discontinuation of ACEI or ARB should be
considered in the setting of either symptomatic hypotension or
uncontrolled hyperkalaemia despite medical treatment, or to reduce
uremic symptoms during treatment of kidney failure (eGFR <15
ml/min/1.73 m2). Mineralocorticoid receptor antagonists (MRA) are
effective for treatment of refractory hypertension, but may cause
hyperkalaemia or reversible decline in kidney function, particularly
in patients with low eGFR [10].
Special recommendation is to avoid any combination of ACEI, ARB, or
direct renin inhibitors (DRI) in patients with CKD, with or without
diabetes. This is a strong recommendation based on evidence from
randomized controlled trials of sufficient duration to evaluate
kidney and CV protection.There is growing evidence that dual RAS
blockade does not lead to long-term CV or kidney benefit despite
lowering proteinuria in the short term, and on the other hand
increases the risks of hyperkalemia and AKI [10]. A large
meta-analysis comparing the effects of monotherapy and dual RAS
blockade in patients with CKD, with and without diabetes, found no
significant differences in all-cause mortality, progression to
end-stage CKD, and CV events between two groups [58]. In contrast,
there is evidence that dual blockade of RAS in patients with CKD,
with and without diabetes, increases the incidence of AKI by 40%
compared to monotherapy [9,59]. Therefore, it can be considered
justified that this recommendation places a higher importance on
preventing hyperkalemia and AKI than on the potential benefits in
reduction of albuminuria [46].
Most patients with CKD will require multiple antihypertensive
therapy with ACEI or ARB in addition to CCB and diuretics to achieve
target BP values. An instrumental variable analysis by Markovitz et
al demonstrated an incremental reduction in SBP and cardiovascular
risk with the addition of each additional antihypertensive agent in
SPRINT [60]. Diuretics are often used in CKD patients with high BP
due to pre-existing hypervolemia, but the literature on the effects
of diuretics on major clinical outcomes in this population is
limited. Limited data have shown that the addition of an MRA, such
as spironolactone, eplerenone, or finerenone, to an ACEI or ARB for
renoprotection in CKD patients reduces blood pressure and urinary
protein/albumin excretion with a quantifiable risk of hyperkalaemia
[61]. The recent FIDELIO-DKD trial (The Finerenone in Reducing
Kidney Failure and Disease Progression in Diabetic Kidney Disease)
in CKD patients with diabetes showed a kidney and cardiovascular
protection by finerenone, despite its modest effect on SBP and
higher incidence of hyperkalemia-related events [62].
CONCLUSION
The updated 2021 KDIGO BP clinical practice guideline insists on
standardized office BP measurement and recommends a target SBP <120
mm Hg in most subpopulations of CKD patients, provided this
technique is used. The implementation of standardized BP measurement
in a busy clinical practice is recognized as a challenge, but is
fundamental to the practice of evidence-based medicine, because the
available evidence for treatment recommendations is derived from the
studies in which BP was measured in this way. That means the
adoption of a target SBP <120 mmHg is inextricably linked to the
technique of standardized office BP measurement, and kidney and
cardiovascular benefits that would result from long-term intensive
BP reduction in patients with CKD depend on it. Given the importance
of these goals and the existing resistance to standardization of the
method, it is possible that the new measures will require the
regulatory enforcement of standardized BP measurement protocols in
routine clinical practice.
Regardless of the recommended target SBP, the KDIGO work group warns
of caution in certain subpopulations of CKD patients, pointing out
that it is reasonable to apply less intensive BP targets in people
with very limited life expectancy or symptomatic orthostatic
hypotension. This suggestion supports physician autonomy and shared
decision making, depending on patient characteristics, tolerability,
and preferences, in order to select patients who are most likely to
benefit from more intensive BP lowering. Large randomized controlled
trials on the effects of intensive BP lowering for cardiovascular,
kidney, and cognitive outcomes and/or survival in CKD patients are
needed, particularly in subpopulations that were not adequately
represented in previous studies. There is also an urgent need for
randomized trials comparing the effects of different combinations of
antihypertensive drugs on outcomes, which would contribute to the
development of evidence-based algorithms for hypertension treatment
in CKD.
REFERENCES
-
Roth GA, Mensah GA, Johnson CO, Addolorato G,
Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular
Diseases and Risk Factors, 1990-2019: Update From the GBD 2019
Study. J Am Coll Cardiol. 2020; 76(25): 2982-3021.
-
Hanratty R, Chonchol M, Havranek EP, Powers JD,
Dickinson LM, Ho PM, et al. Relationship between blood pressure
and incident chronic kidney disease in hypertensive patients.
Clin J Am Soc Nephrol. 2011; 6(11): 2605-11.
-
Muntner P, Anderson A, Charleston J, Chen Z,
Ford V, Makos G, et al. Hypertension awareness, treatment, and
control in adults with CKD: results from the Chronic Renal
Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010; 55(3):
441-51.
-
Johansen KL, Chertow GM, Foley RN, Gilbertson
DT, Herzog CA, Ishani A, et al. US Renal Data System 2020 Annual
Data Report: Epidemiology of Kidney Disease in the United
States. Am J Kidney Dis. 2021; 77 (4 Suppl 1): A7-A8.
-
SPRINT research group, Wright JT Jr, Williamson
JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A
Randomized Trial of Intensive versus Standard Blood-Pressure
Control. N Engl J Med. 2015; 373(22): 2103-16.
-
Soliman EZ, Ambrosius WT, Cushman WC, Zhang ZM,
Bates JT, Neyra JA, et al; SPRINT Research Study Group. Effect
of Intensive Blood Pressure Lowering on Left Ventricular
Hypertrophy in Patients With Hypertension: SPRINT (Systolic
Blood Pressure Intervention Trial). Circulation. 2017; 136(5):
440-50.
-
Kidney Disease: Improving Global Outcomes
(KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice
Guideline for the Management of Blood Pressure in Chronic Kidney
Disease. Kidney Int Suppl. 2012; 2: 337–414.
-
Beddhu S, Chertow GM, Greene T, Whelton PK,
Ambrosius WT, Cheung AK, et al. Effects of Intensive Systolic
Blood Pressure Lowering on Cardiovascular Events and Mortality
in Patients With Type 2 Diabetes Mellitus on Standard Glycemic
Control and in Those Without Diabetes Mellitus: Reconciling
Results From ACCORD BP and SPRINT. J Am Heart Assoc. 2018;
7(18): e009326.
-
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner
TA, Duckworth W, et al. VA NEPHRON-D Investigators. Combined
angiotensin inhibition for the treatment of diabetic
nephropathy. N Engl J Med. 2013; 369(20): 1892-903.
-
Kidney Disease: Improving Global Outcomes
(KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice
Guideline for the Management of Blood Pressure in Chronic Kidney
Disease. Kidney Int. 2021; 99(3S): S1-S87.
-
Riva-Rocci S Un nuovo sfigmomanometro. Gazz Medi
Torino. 1896; 50: 981–96.
-
Korotkoff NC. To the question of methods of
determining the blood pressure. Rep Imp Military Acad. 1905; 11:
365–36.
-
Roerecke M, Kaczorowski J, Myers MG. Comparing
automated office blood pressure readings with other methods of
blood pressure measurement for identifying patients with
possible hypertension: a systematic review and meta-analysis.
JAMA Intern Med. 2019; 179(3): 351–62.
-
Drawz PE, Agarwal A, Dwyer JP, Horwitz E, Lash
J, Lenoir K, et al. Concordance Between Blood Pressure in the
Systolic Blood Pressure Intervention Trial and in Routine
Clinical Practice. JAMA Intern Med. 2020; 180(12): 1655-63.
-
Whelton PK, Carey RM, Aronow WS, Casey DE Jr,
Collins KJ, Dennison Himmelfarb C, et al. 2017
ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for
the Prevention, Detection, Evaluation, and Management of High
Blood Pressure in Adults: executive summary: a report of the
American College of Cardiology/American Heart Association Task
Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;
71(19): 2199–269.
-
Williams B, Mancia G, Spiering W, Agabiti Rosei
E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the
management of arterial hypertension. Eur Heart J. 2018; 39(33):
3021–104.
-
Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla
M, Ahmed SB, Dumanski SM, et al. Hypertension Canada's 2020
Comprehensive Guidelines for the Prevention, Diagnosis, Risk
Assessment, and Treatment of Hypertension in Adults and
Children. Can J Cardiol. 2020; 36(5): 596-624.
-
Muntner P, Shimbo D, Carey RM, Charleston JB,
Gaillard T, Misra S, et al. Measurement of blood pressure in
humans: a scientific statement from the American Heart
Association. Hypertension. 2019; 73(5): e35–e66.
-
Duncombe SL, Voss C, Harris KC. Oscillometric
and auscultatory blood pressure measurement methods in children:
a systematic review and meta-analysis. J Hypertens. 2017; 35:
213-24.
-
Mingji C, Onakpoya IJ, Heneghan CJ, Ward AM.
Assessing agreement of blood pressure-measuring devices in
Tibetan areas of China: a systematic review. Heart Asia. 2016;
8: 46-51.
-
Johnson KC, Whelton PK, Cushman WC, Cutler JA,
Evans GW, Snyder JK, et al. Blood pressure measurement in SPRINT
(Systolic Blood Pressure Intervention Trial). Hypertension.
2018; 71(5): 848–85.
-
Kollias A, Stambolliu E, Kyriakoulis KG,
Gravvani A, Stergiou GS. Unattended versus attended automated
office blood pressure: systematic review and meta-analysis of
studies using the same methodology for both methods. J Clin
Hypertens (Greenwich). 2019; 21(2): 148–55.
-
Drawz PE, Brown R, De Nicola L, Fujii N, Gabbai
FB, Gassman J, et al; CRIC Study Investigators. Variations in
24-Hour BP Profiles in Cohorts of Patients with Kidney Disease
around the World: The I-DARE Study. Clin J Am Soc Nephrol. 2018;
13(9): 1348-57.
-
Drawz PE, Alper AB, Anderson AH, Brecklin CS,
Charleston J, Chen J, et al; Chronic Renal Insufficiency Cohort
Study Investigators. Masked Hypertension and Elevated Nighttime
Blood Pressure in CKD: Prevalence and Association with Target
Organ Damage. Clin J Am Soc Nephrol. 2016; 11(4): 642-52.
-
Minutolo R, Gabbai FB, Agarwal R, Chiodini P,
Borrelli S, Bellizzi V, et al. Assessment of achieved clinic and
ambulatory blood pressure recordings and outcomes during
treatment in hypertensive patients with CKD: a multicenter
prospective cohort study. Am J Kidney Dis. 2014; 64(5): 744-52.
-
Cook NR, Appel LJ, Whelton PK. Lower levels of
sodium intake and reduced cardiovascular risk. Circulation.
2014; 129(9): 981-9.
-
Filippini T, Malavolti M, Whelton PK, Naska A,
Orsini N, Vinceti M. Blood Pressure Effects of Sodium Reduction:
Dose-Response Meta-Analysis of Experimental Studies.
Circulation. 2021; 143(16): 1542-67.
-
McMahon EJ, Campbell KL, Bauer JD, Mudge DW,
Kelly JT. Altered dietary salt intake for people with chronic
kidney disease. Cochrane Database Syst Rev. 2021; 6(6):
CD010070.
-
Lambers Heerspink HJ, Holtkamp FA, Parving HH,
Navis GJ, Lewis JB, Ritz E, et al. Moderation of dietary sodium
potentiates the renal and cardiovascular protective effects of
angiotensin receptor blockers. Kidney Int. 2012; 82(3): 330-7.
-
World Health Organization. Guideline: sodium
intake for adults and children. World Health Organization; 2012.
-
Kidney Disease: Improving Global Outcomes
(KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice
Guideline for Diabetes Management in Chronic Kidney Disease.
Kidney Int. 2020; 98(4S): S1-S115.
-
Flesher M, Woo P, Chiu A, Charlebois A,
Warburton DE, Leslie B. Self-management and biomedical outcomes
of a cooking, and exercise program for patients with chronic
kidney disease. J Ren Nutr. 2011;21(2):188-95.
-
Heiwe S, Jacobson SH. Exercise training in
adults with CKD: a systematic review and meta-analysis. Am J
Kidney Dis. 2014; 64(3): 383-93.
-
Beddhu S, Wei G, Marcus RL, Chonchol M, Greene
T. Light-intensity physical activities and mortality in the
United States general population and CKD subpopulation. Clin J
Am Soc Nephrol. 2015; 10(7): 1145-53.
-
Hallan SI, Rifkin DE, Potok OA, Katz R, Langlo
KA, Bansal N, et al. Implementing the European Renal Best
Practice Guidelines suggests that prediction equations work well
to differentiate risk of end-stage renal disease vs. death in
older patients with low estimated glomerular filtration rate.
Kidney Int. 2019; 96: 728-37.
-
Cheung AK, Rahman M, Reboussin DM, Craven TE,
Greene T, Kimmel PL, et al; SPRINT Research Group. Effects of
intensive BP control in CKD. J Am Soc Nephrol. 2017; 28:
2812-23.
-
Pajewski NM, Berlowitz DR, Bress AP, Callahan
KE, Cheung AK, Fine LJ, et al. Intensive vs standard blood
pressure control in adults 80 years or older: a secondary
analysis of the Systolic Blood Pressure Intervention Trial. J Am
Geriatr Soc. 2020; 68: 496-504.
-
Bress AP, King JB, Kreider KE, Beddhu S, Simmons
DL, Cheung AK, et al; SPRINT Research Group. Effect of intensive
versus standard blood pressure treatment according to baseline
prediabetes status: a post hoc analysis of a randomized trial.
Diabetes Care. 2017; 40: 1401-8.
-
Blood Pressure Lowering Treatment Trialists'
Collaboration, Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi
F, Cass A, et al. Blood pressure lowering and major
cardiovascular events in people with and without chronic kidney
disease: meta-analysis of randomised controlled trials. BMJ.
2013; 347: f5680.
-
Ettehad D, Emdin CA, Kiran A, Anderson SG,
Callender T, Emberson J, et al. Blood pressure lowering for
prevention of cardiovascular disease and death: a systematic
review and meta-analysis. Lancet. 2016; 387(10022): 957-67.
-
Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW,
Collins AJ, et al. The effect of a lower target blood pressure
on the progression of kidney disease: long-term follow-up of the
Modification of Diet in Renal Disease Study. Ann Intern Med.
2005; 142: 342-51.
-
Upadhyay A, Earley A, Haynes SM, Uhlig K.
Systematic review: blood pressure target in chronic kidney
disease and proteinuria as an effect modifier. Ann Intern Med.
2011; 154: 541-8.
-
Cushman WC, Evans GW, Byington RP, Goff DC Jr,
Grimm RH Jr, Cutler JA, et al; ACCORD Study Group. Effects of
intensive blood-pressure control in type 2 diabetes mellitus. N
Engl J Med. 2010; 362: 1575-85.
-
Papademetriou V, Zaheer M, Doumas M, Lovato L,
Applegate WB, Tsioufis C, et al; ACCORD Study Group.
Cardiovascular outcomes in action to control cardiovascular risk
in diabetes: impact of blood pressure level and presence of
kidney disease. Am J Nephrol. 2016; 43: 271-80.
-
Beddhu S, Greene T, Boucher R, Cushman WC, Wei
G, Stoddard G, et al. Intensive systolic blood pressure control
and incident chronic kidney disease in people with and without
diabetes mellitus: secondary analyses of two randomised
controlled trials. Lancet Diabetes Endocrinol. 2018; 6(7):
555-63.
-
Drawz PE, Beddhu S, Bignall ONR 2nd, Cohen JB,
Flynn JT, Ku E, et al. KDOQI US Commentary on the 2021 KDIGO
Clinical Practice Guideline for the Management of Blood Pressure
in CKD. Am J Kidney Dis. 2022; 79(3): 311-27.
-
National Institute for Health and Care
Excellence. Hypertension in adults: diagnosis and management.
NICE guideline [NG136]. Available at:
https://www.nice.org.uk/guidance/ng136. Accessed January 11,
2021.
-
Visseren FLJ, Mach F, Smulders YM, Carballo D,
Koskinas KC, Bäck M, et al; ESC National Cardiac Societies; ESC
Scientific Document Group. 2021 ESC Guidelines on cardiovascular
disease prevention in clinical practice. Eur Heart J. 2021;
42(34): 3227-37.
-
Maschio G, Alberti D, Locatelli F, Mann JF,
Motolese M, Ponticelli C, et al. Angiotensin-converting enzyme
inhibitors and kidney protection: the AIPRI trial. The ACE
Inhibition in Progressive Renal Insufficiency (AIPRI) Study
Group. J Cardiovasc Pharmacol. 1999; 33 (Suppl 1): S16-20.
-
Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang
WR, et al. Efficacy and safety of benazepril for advanced
chronic renal insufficiency. N Engl J Med. 2006; 354(2): 131-40.
-
Randomised placebo-controlled trial of effect of
ramipril on decline in glomerular filtration rate and risk of
terminal renal failure in proteinuric, non-diabetic nephropathy.
The GISEN Group (Gruppo Italiano di Studi Epidemiologici in
Nefrologia). Lancet. 1997; 349(9069): 1857-63.
-
Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S.
Renal insufficiency as a predictor of cardiovascular outcomes
and the impact of ramipril: the HOPE randomized trial. Ann
Intern Med. 2001; 134(8): 629-36.
-
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl
MA, Lewis JB, et al; Collaborative Study Group. Renoprotective
effect of the angiotensin-receptor antagonist irbesartan in
patients with nephropathy due to type 2 diabetes. N Engl J Med.
2001; 345(12): 851-60.
-
Brenner BM, Cooper ME, de Zeeuw D, Keane WF,
Mitch WE, Parving HH, et al; RENAAL Study Investigators. Effects
of losartan on renal and cardiovascular outcomes in patients
with type 2 diabetes and nephropathy. N Engl J Med. 2001;
345(12): 861-9.
-
Gerstein HC, Mann JF, Pogue J, Dinneen SF, Hallé
JP, Hoogwerf B, et al. Prevalence and determinants of
microalbuminuria in high-risk diabetic and nondiabetic patients
in the Heart Outcomes Prevention Evaluation Study. The HOPE
Study Investigators. Diabetes Care. 2000; 23 (Suppl 2): B35-9.
-
Mann JF, Gerstein HC, Yi QL, Franke J, Lonn EM,
Hoogwerf BJ, et al; HOPE Investigators. Progression of renal
insufficiency in type 2 diabetes with and without
microalbuminuria: results of the Heart Outcomes and Prevention
Evaluation (HOPE) randomized study. Am J Kidney Dis. 2003;
42(5): 936-42.
-
Rahman M, Ford CE, Cutler JA, Davis BR, Piller
LB, Whelton PK, et al; ALLHAT Collaborative Research Group.
Long-term renal and cardiovascular outcomes in Antihypertensive
and Lipid-Lowering Treatment to Prevent Heart Attack Trial
(ALLHAT) participants by baseline estimated GFR. Clin J Am Soc
Nephrol. 2012; 7(6): 989-1002.
-
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou
W, et al. Renin-Angiotensin System Inhibitors and Kidney and
Cardiovascular Outcomes in Patients With CKD: A Bayesian Network
Meta-analysis of Randomized Clinical Trials. Am J Kidney Dis.
2016; 67(5): 728-41.
-
Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig
A, Wang X, et al; ONTARGET and TRANSCEND Investigators.
Cardiovascular and renal outcomes with telmisartan, ramipril, or
both in people at high renal risk: results from the ONTARGET and
TRANSCEND studies. Circulation. 2011; 123(10): 1098-107.
-
Markovitz AA, Mack JA, Nallamothu BK, Ayanian
JZ, Ryan AM. Incremental effects of antihypertensive drugs:
instrumental variable analysis. BMJ. 2017; 359: j5542.
-
Currie G, Taylor AH, Fujita T, Ohtsu H,
Lindhardt M, Rossing P, et al. Effect of mineralocorticoid
receptor antagonists on proteinuria and progression of chronic
kidney disease: a systematic review and meta-analysis. BMC
Nephrol. 2016; 17(1): 127.
-
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope
LM, Rossing P, et al; FIDELIO-DKD Investigators. Effect of
Finerenone on Chronic Kidney Disease Outcomes in Type 2
Diabetes. N Engl J Med. 2020; 383(23): 2219-29.
-
Unger T, Borghi C, Charchar F, Khan NA, Poulter
NR, Prabhakaran D, et al. 2020 International Society of
Hypertension Global Hypertension Practice Guidelines.
Hypertension. 2020; 75(6): 1334-57.
|
|
|
|





