| |
|
|
INTRODUCTION
Corona Virus Disease 2019 (COVID-19) has brought the life of the
whole humanity to a standstill. Catastrophic loss of life, a
confusion in healthcare and the vulnerability of the global economy
are some of the outcomes of this pandemic. COVID-19 infection
affects global population regardless of age and gender, and with
comorbidities present, COVID-19 and its complications escalate at an
alarming rate. Cardiovascular (CVD) diseases per se are the leading
cause of death globally with an estimated 31% of deaths worldwide of
which nearly 85% are due to heart attack and stroke. Scientific
researchers have noted that individuals with pre-existing CV
diseases and conditions are relatively more susceptible to infection
with COVID-19 [1,2]. Moreover, it was shown in the comparison
between subgroups: milder and more severe cases, survivors and
non-survivors, patients from intensive care units and those who were
not in intensive care [2]. The impact of the COVID-19 preventive
measures of isolation and quarantine (lockdown) on CVD patients in
Denmark showed that at that time, compared to the pre-Covid 19 era,
there was no difference in the mortality of CVD patients. However,
an increased out-of-hospital mortality and decreased in-hospital
mortality were found. In contrast, in Germany and France, there was
a significant increase in mortality, even by 12-20% in CV patients
in April 2021.
Strategies for the diagnosis of SARS-CoV-2
The diagnosis of COVID-19 is based on a combination of
epidemiological criteria (contact within the incubation period), the
presence of clinical symptoms, laboratory tests (PCR tests) and
tests based on clinical imaging. Antibody-based tests and SARS-CoV-2
antigen enzyme-linked immunosorbent assay (ELISA) are under
development and not yet fully validated. Widespread testing has
proven effective in the containment phase of the epidemic. The
quality of sample collection (deep nasal swab) and transport (time)
to the laboratory is necessary to avoid false negative results. Lung
computed tomography (MSCT) can be used as a diagnostic test for
COVID 19 [3] .
We know that the penetration of the SARS COV-2 virus and the cause
of the COVID-19 infection, after a short incubation and various
respiratory symptoms, loss of the sense of smell and general
symptoms: elevated body temperature, malaise, myalgia and arthralgia,
most often affect the lung parenchyma . At the beginning lung damage
manifests like flu syndrome (cough and fever), which is progressing
to the pneumonia (dyspnea, hypoxemia , tachypnea ) and , in some
cases , to _ acute respiratory distress syndrome or non-cardiogenic
pulmonary edema ( ARDS ).
Acute cardiac lesion is an ordinary extrapulmonary manifestation of
COVID- 19 with potential chronic consequences. Clinical
manifestations include directcardiac involvement and mechanisms of
indirect immune response which affect the cardiovascular system and
implications on the treatment of patients after the recovery from
the acute COVID- 19 infections [4]. Early radiography of the lungs
and the heart and the most reliable MSCT (multilayer, multidetector
computer-tomographic scan) of the thorax show detectable changes in
the lung parenchyma in up to 85% of patients, which can be both
oligosymptomatic and asymptomatic [5].
PATHOGENESIS OF ACUTE COVID-19 MYOCARDIAL LESIONS
Acute COVID-19 myocardial lesion whose marker is elevated
high-sensitivity troponin T is present in > 12% of infected patients
[6]. Hence, cardiac lesions in patients infected with the SARS COV-2
virus become associated with higher morbidity and mortality. [6].
Severe acute respiratory distress syndrome-caused by coronavirus 2
(SARS-CoV-2) is manifested by the dominance of excessive production
of inflammatory cytokines (IL-6 and TNF-α), which leads to systemic
inflammation and syndrome of multiple dysfunction of organ systems,
acutely involving the cardiovascular system. Hypertension (56.6%)
and diabetes (33.8%) are the most common comorbidities in those
infected with COVID-19 who require hospitalization. Cardiac lesion,
defined as elevated high-sensitivity troponins T and I, is
significantly correlated with inflammatory biomarkers: interleukins
6 and 2 (IL-6, IL- 2) and C-reactive protein (hsCRP),
hyperferritinemia and leukocytosis, and reflects a significant
association of the myocardial lesion and inflammatory hyperactivity
caused by viral infection [6]. In addition, mechanisms by which
activated platelets intensify pre-existing endothelial activation
and dysfunction, most likely caused by the release of
platelet-derived calcium-binding proteins (SA 100A8 and SA 100A9),
have been described. Coronavirus 2 (SARS-CoV-2), the etiological
agent of COVID-19, can infect the heart, vascular tissues and
circulating cells via ACE2 (angiotensin-converting enzyme 2), the
host cell receptor for the viral spike protein. Endotheliitis caused
by SARS-CoV-2 [1] involves the interaction of the viral spike
(S-protein part of the virus, the so-called spike) protein with the
endothelial enzyme that converts angiotensin 2 (ACE2 convertase)
together with alternative mechanisms via nucleocapsids and
viroporins. These events create a cycle of intravascular
inflammation and coagulation driven by the SARS-CoV-2 virus, which
significantly contributes to poor clinical outcome in patients with
more severe forms of infection. Patients with risk factors and/or
cardiovascular diseases are prone to developing severe forms of
COVID-19 and its complications (FIGURE 1). The host's response to
the virus leads to signs of systemic inflammation, with increases in
markers of inflammation (hsCRP, procalcitonin, d-dimer, IL-6,
ferritin, LDH) and myocardial lesions and/or cardiac dysfunction (troponin
and/or NT-proBNP), which predisposes to acute heart failure,
myocarditis, thrombosis and arrhythmias. These CV complications
interfere with the host's response to the virus, which can lead to
shock syndrome, multiple organ failure, and death [7]. (FIGURE 1)
FIGURE 1. Corona virus and the heart.
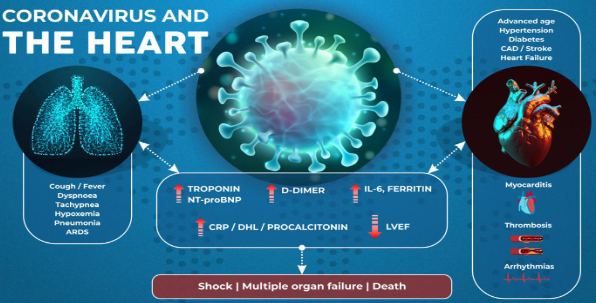
LEGEND: CAD: coronary artery disease; LDH: lactate dehydrogenase;
LVEF: left ventricular ejection fraction; CRP: C-reactive protein;
IL-6: interleukin-6; ARDS: acute respiratory distress syndrome [7].
retrieved from
https://abccardiol.org/wp-content/uploads/articles_xml/0066-782X-abc-20200279/0066-782X-abc-20200279-en.pdf
COVID-19 AND CARDIOVASCULAR COMORBIDITIES
A meta-analysis of 6 studies from China with 72314 COVID-19
patients shows a high prevalence of arterial hypertension (17 ± 7%),
diabetes mellitus (8 ± 6%) and cardiovascular disease (CVD) (5 ± 4%)
as comorbidities [7, 8]. In 138 hospitalized patients with COVID-19
and pneumonia, Wang et al found a high prevalence of hypertension
(31.2%), CVD (19.6%), and diabetes (10.1%), and these comorbidities
lead to the most severe forms of COVID 19 which usually requires
hospitalization (hypoxemia, need for treatment in intensive care),
especially in the elderly (median 42-64 years old) [9]. Another
meta-analysis of 7 studies, on 1576 out-of-hospital infected
patients, shows the highest prevalence of comorbidities:
hypertension (21.1%), diabetes (9.7%), cardiovascular diseases (CVD)
(8.4%) and chronic respiratory diseases (1 .5%). By comparing severe
forms of COVID-19 with moderate and mild ones, a statistical
parameter was obtained: ODDS ratio (OR) - odds ratio for a bad
outcome: for hypertension - 2.36 (95% CI: 1.46–3.83), for
respiratory diseases – 2.46 (95% CI: 1.76–3.44) and the highest for
cardiovascular diseases - 3.42 (95% CI: 1.88–6.22)/respectively
[10].
MORTALITY IN RELATION TO PREVIOUSLY RELEVANT CHRONIC DISEASES
An analysis of 72314 confirmed cases of COVID-19 (Wuhan) found a
total mortality of 1663 patients or 2.3%, with the presence of a
previous disease: 10.5% with CV disease, 7.3% with diabetes mellitus
and 6% with arterial hypertension. Cardiovascular complications due
to COVID-19 associated with comorbidities were: myocardial lesion
(20%), cardiac arrhythmias (16%), myocarditis (10%) and acute heart
failure and cardiogenic shock (about 5%) [8,9,11, 12]. Guo et al,
evaluating a cohort of 187 patients, found that those with
myocardial lesions had a higher prevalence of hypertension (63% vs
28%), diabetes (30.8% vs 8.9%), coronary disease (32.7% vs 3% ) and
heart failure (15.4% vs 0%) and these are of older age (median 71.4
years) [9]. In a group of 191 patients, Zhou et al. compared those
discharged from the hospital with those who died and those who died
had a higher prevalence of hypertension (48%), diabetes (31%) and
CVD (24%) [13].
CARDIOVASCULAR DISEASE IN PATIENTS WITH COVID-19
COVID-19 patients treated in the intensive care unit had the
following diagnoses from which they died: acute respiratory distress
syndrome (ARDS) in 61%, severe cardiac arrhythmias in 44% and shock
syndrome in 31%. Some autopsy findings suggested myocardial
infiltration by mononuclear leukocytes and revealed some cases of
severe myocarditis with a dilated phenotype [14,15]. COVID-19, as
well as earlier coronaviruses and influenza epidemics, suggest an
association with acute coronary events, arrhythmias and exacerbation
of chronic heart failure, but the data also suggest the development
of DE NOVO cases of cardiovascular diseases and worsening of the
existing ones [14]. Cardiac lesion in patients infected by SARS COV
-2 virus (COVID -19) is associated with higher risk from: myocardial
infarction, fulminant myocarditis which quickly develops with
lowered EF left ventricular function, arrhythmias, venous
thromboembolism, cardiomyopathy which reminds of the acute heart
attack with ST elevation - STEMI the so-called Takotzubo
cardiomyopathy. In addition, SARS-CoV-2 tropism and interaction with
the rennin-angiotensin-aldosterone system (RAAS), through the ACE2
receptor, enhances the inflammatory response and aggression to the
heart, leading to the imperative position on the use of ACE
inhibitors and angiotensin receptor blockers (ARBs, sartans) in
infected patients. CV consequences lead to a poor prognosis,
emphasizing the importance of their early detection and the
introduction of an optimal treatment strategy [6]. Among
hospitalized patients with COVID-19, evidence of acute impairment of
cardiac function is common and includes the following: acute heart
failure (3%–33%), cardiogenic shock (9%–17%), myocardial ischemia or
infarction (0.9% -11%), ventricular dysfunction (left ventricular
[10%–41%], right ventricular [33%–47%], biventricular [3%–15%]),
stress cardiomyopathy (2%–5.6%), arrhythmias (9%–17%), venous
thromboembolism (23%–27%) and arterial thrombosis secondary to
viral-mediated coagulopathy [4]. A Danish study based on a national
registry of over 5000 hospitalized patients with COVID-19 found that
the risk of acute MI and ischemic stroke was 5-fold and 10-fold
higher, respectively, during the first 14 days after infection with
COVID-19 compared with the period which preceded the known infection
[16].
PROGNOSIS OF CVS DAMAGE IN COVID-19 AND PREDICTORS OF MORTALITY
The prognosis depends on the presence of CV risk factors (e.g.
male gender, older age, population, hypertension, diabetes),
comorbidities (e.g. coronary disease and other cardiac diseases,
chronic obstructive pulmonary disease, chronic renal failure and
malignancies) that predispose patients with COVID-19 to more severe
forms of diseases and increased mortality [4]. Racial and ethnic
disparities in the outcomes of COVID -19 are also evident [4].
Advanced age is an independent predictor of mortality in COVID-19
infection. The mortality rate increases with age as follows: 1.3% in
patients aged 50-59 years; 3.6% in patients aged 60-69; 8% in
patients aged 70-79 years; and 14.8% in patients older than 80
years. Population studies have reported an overall mortality rate of
6% in patients with hypertension, 7.3% in patients with diabetes,
and 10.5% in patients with CVD. Patients with malignant tumors have
a higher risk of COVID-19 due to impaired immune defenses and the
consequences of antineoplastic treatment. In China, among confirmed
cases of COVID-19, the prevalence of cancer ranged from 1% to 7%,
which is higher than the total incidence of cancer in that country
(0.2%). Patients with cancer were more likely to develop a severe
form of COVID-19 compared to those without cancer (39% vs. 8%). Of
cancer patients who had undergone recent chemotherapy or surgery,
75% developed severe disease compared with 3% of those who had not
received recent treatment [17].
Biomarker evidence of cardiac lesion is strongly associated with
worse outcomes in COVID-19. Elevation of cardiac biomarkers, such as
NT-proBNP, Troponin(Tn) T and I or D-dimer, predicts poor clinical
outcomes. In hospitalized patients with COVID-19, the prevalence of
elevated hs-TnT (high-sensitivity troponin-T) is 20% to 30%. Based
on such elevated Tn levels, acute myocardial lesions range from 8%
to 62% according to various data, and more severe forms of the
disease are associated with higher levels of cardiac biomarkers.
Elevated Tn levels were rare in survivors of uncomplicated COVID-19
(1%–20%), common in critically ill patients (46%–100%), and almost
universally elevated in critically ill (ie, requiring intensive care
or mechanical ventilation and those who did not survive) [11].
Among 2736 hospitalized patients with COVID-19 in New York, even
small elevations of Troponin I (>0.03–0.09 ng/mL) were associated
with higher mortality.
DAMAGE to the myocardium and earlier CVD
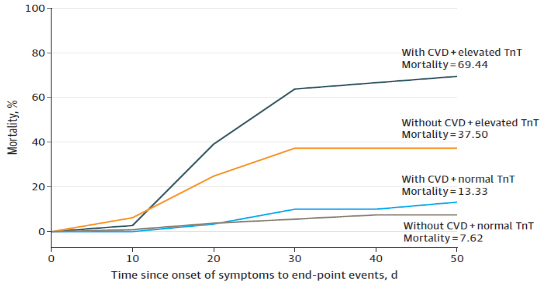
Data of a retrospective study, of COVID-19 patients hospitalized in
7 hospitals in Wuhan in the period 23.01.-23.02.2020 [ 18].
Moreover, the greater the increase in TnT, the greater the risk
of mortality [18]. Compared with those without elevated TnI,
COVID-19 patients with elevated Tn have a higher risk of acute
respiratory distress syndrome (58%–59% vs. 12%–15%), need for
mechanical ventilation (22%–60% vs. 4% –10%), malignant arrhythmias
(17% vs 2% VT/VF) and death (51%–95% vs 5%–27%). Tn and NT-proBNP
levels increased during hospitalization in non-survivors but not in
survivors [11,13].
VISUALIZATION METHODS IN PROVING MYOCARDIAL LESIONS
COVID-19 is associated with abnormalities of cardiac structure
and function including echocardiographic evidence of left
ventricular dysfunction, regional wall motion abnormalities, and
mild reduction in right ventricular function [19] . Several
cardiovascular magnetic resonance (CMR) studies have documented
myocardial abnormalities that persist after acute infection. In a
study of 100 patients with COVID-19, imaging was performed an
average of 71 days after the diagnosis of COVID-19. Pericardial
effusion (>10 mm) was detected in 20% (20/100) of patients. Late
gadolinium enhancement (LGE), reflecting fibrosis and cicatrix, was
observed in 32% and was significantly more common in patients with
COVID-19 than in healthy or risk-factor-matched controls. In
addition, other studies have noted a high prevalence of myocardial
edema following COVID-19 infection. Whether the abnormal CMR imaging
findings observed after COVID-19 reflect a permanent cardiac lesion
is unknown at this time due to the lack of long-term studies.
RADIOGRAPHY AND MSCT OF THE CHEST
Early MSCT (multislice, multidetector computed tomography scan)
of the thorax shows detectable changes in the lung parenchyma in as
many as 85% of patients, which can be both oligosymptomatic and
asymptomatic. Also, in as many as 75%, there are COVID-19 bilateral
lung changes with subpleural and peripheral distribution [5]. In
addition to other viral pneumonias, COVID-19 pneumonia on the Rtg
manifests as peripherally located ground glass opacity. Perihilar or
diffuse widespread ground-glass opacification and ‘’crazy paving"
are present in MSCT findings in COVID- 19 and are difficult to
distinguish from the others diseases only on the basis CT findings
(other viral pneumonia, acute respiratory distress syndrome - ARDS,
acute hypersensitive pneumonitis, sarcoidosis, pulmonary hemorrhage,
alveolar proteinosis) [20,21].
By appearance, peripherally located consolidations with marginal
zone ground glass opacity do not differ from the findings in
Cryptogenic organizing C organizing_ pneumonia ( COP ), Eosinophilic
pneumonia , Vasculitis , Invasive aspergillosis and should be
interpreted within the whole clinical picture. The organizing
pneumonia (pulmonary tissue consolidation) in COVID- 19 has the same
characteristics as the organizing pneumonias of other causes .
Nodules with a halo sign, apart from COVID-19, are also a common
finding in numerous other diseases. [20,21].
Even in less severe, ambulatory-treated COVID-19 patients, signs of
incipient lung congestion can be detected on a chest radiograph:
Kerley B lines and redistribution of the pulmonary vascular pattern.
In patients who are treated in intensive care units, enlargement of
the cardiac shadow-cardiomegaly, bilateral pleural effusion as part
of cardiac decompensation and pronounced lung congestion can be
detected. MSCT is sovereign in the detection of thrombus in the
branches of the pulmonary artery and the diagnosis of pulmonary
thromboembolism [16]
ABNORMALITIES INDICATING A HEART LESION ON ECHOCARDIOGRAPHY
Echocardiography - (ultrasound of the heart) is the most
accessible method that can also be performed as an emergency at the
patient's bedside (point of care-POC approach). Echocardiographic
abnormalities commonly registered in hospitalized patients with
COVID-19 include right ventricular (RV) dysfunction (26.3%), left
ventricular (LV) wall motion abnormalities (23.7%), global left
ventricular dysfunction with reduced LV EF (18.4%), grade II or III
diastolic dysfunction (13.2%) and pericardial effusion (7.2%) [22].
Biomarker evidence of myocardial lesion associated with
echocardiographic abnormalities correlates with a higher risk of
in-hospital mortality. Myocardial involvement caused by SARS-CoV-2
infection may be important for long-term prognosis. Myocardial
effects during SARS-CoV-2 infections can be characterized with
advanced echocardiographic techniques. Strain imaging was performed
in 18 patients with SARS CoV-2 infection assessing longitudinal,
radial, and circumferential strain or left ventricular (LV) strain
including rotation, torsion, and twisting [17]. LV deformation
(strain) was also analyzed in a control group of healthy individuals
of the appropriate age (n = 20). The dominant finding was the
finding: reduced longitudinal strain observed predominantly in more
than one basal segment of the LV (n = 10/14 patients, 71%). This
pattern resembles a "reverse Tako-tsubo" morphology, which is not
typical of other viral myocarditis. Additional findings included a
biphasic pattern with maximal postsystolic thickening or negative
regional radial strain predominantly in the basal segments (n = 5/14
patients, 36%); absence or dispersion of left ventricular basal
rotation (n = 6/14 patients, 43%); decreased or positive regional
circumferential strain in more than one segment (n = 7/14 patients,
50%); net rotation showing late post-systolic twist or biphasic
pattern (n = 8/14 patients, 57%); cardiac rotation showing a
polyphasic pattern and/or higher peak values during diastole (n =
8/14 patients, 57%). Descriptive myocardial damage due to
SARS-CoV-2-infection was highly prevalent in the presented cohort,
even in patients with mild symptoms. COVID-19 myocardial damage
appears to be characterized by specific deformation (strain)
abnormalities in the basal segments of the LV. These data raise an
idea for prospective testing: whether these parameters are useful
for risk stratification and for long-term follow-up of these
patients [17].
It is important to present a large meta -analysis by Ogungbe O. et
al [23] on 41013 pa-tients, where the aim was to quantify the
relation-ship between myocardial lesion biomarkers, co-agulation and
severe COVID-19 and death in hos-pitalized patients. Individual
study effect esti-mates of the association of markers of myocardial
lesion (troponins), myocardial dysfunction (N-terminal-prohormone
BNP, NT-proBNP) and coag-ulopathy (D-dimer) and death or
severe/critical COVID-19 were pooled using the statistical pa-rameter
Odds ratio (odds ratios for adverse events-OR) by outcomes of
critical/severe COVID-19 and death. Comorbidities of hypertension -
39% (95% CI: 34–44%); diabetes, - 21% (95% CI: 18%–24%); coronary
artery disease, 13% (95% CI: 10–16%); chronic obstructive pulmonary
dis-ease, 7% (95% CI: 5–8%); and history of malig-nancy, 5% (95% CI:
4–7%). Elevated troponin was associated with higher pooled odds of
criti-cal/severe COVID-19 and death [OR: 1.76, 95% (CI: 1.42–2.16)];
By separate OR analysis, the odds ratio for death was OR: 1.72, 95%
(CI: 1.32–2.25) and for critical/severe COVID-19, OR: 1.93, 95% (
CI: 1 ,45–2,40). Elevations of NT-proBNP were also associated with
more severe COVID-19 and death (OR: 3.00, 95% CI: 1.58–5.70).
In-creased D-dimer levels were significantly associ-ated with
critical/severe COVID-19 and death (pooled OR: 1.38, 95% CI:
1.07–1.79). This meta-analysis synthesizes the existing evidence
that myocardial injury and coagulopathy are signifi-cant
complications of COVID-19. The reversibility and functional
significance of these complications and their contribution to
long-term cardiac dis-ease outcomes are still being investigated.
Pa-tients who have recovered from COVID-19 may benefit from
assessment of markers of myocardial injury, heart
dysfunction-failure, and coagulopathy for early risk stratification
[23].
An important aspect of COVID-19 pandemic is the associated
collateral damage in the treatment of many other diseases. This
includes diagnostic difficulty and treatment of all forms of cardiac
and other serious chronic diseases of other organ systems and not
only the treatment of infarctions and acute cardiac diseases during
the COVID-19 pandemic, which has consequences for our daily
cardiology practice. [24,25]
ABNORMALITIES INDICATING A LESION ON CARDIAC MAGNETIC RESONANCE
(CMR)
CMR findings include: T1 mapping abnormalities (suggesting
diffuse myocardial changes such as diffuse fibrosis and/or edema);
T2 mapping abnormalities (more specific to myocardial inflammation,
as occurs in acute myocarditis); the presence of late gadolinium
enhancement (LGE), which indicates an acute myocardial lesion and/or
myocardial fibrosis); or pericardial involvement – all of which may
indicate cardiac lesions associated with COVID-19. In a systematic
review of 199 patients, post-recovery CMR studies in patients with
COVID-19, CMR diagnosed myocarditis in 40.2%, myopericarditis in
1.5%, Takotsubo in 1.5%, ischemia in 2.5% and a double lesion:
ischemia and non-ischemic changes in 2.0%. Regional wall motion
abnormalities were reported in 40.6%, edema (on T2 or short tau
inversion recovery) in 51.1%, LGE in 42.7%, and T1 and T2 mapping
abnormalities in 73% and 63%, respectively. Additionally, perfusion
and extracellular volume mapping abnormalities were described in 85%
and 52% of patients, respectively. Pericardial involvement included
pericardial effusion in 24% and pericardial LGE in 22%. In summary,
the most common CMR diagnosis in COVID-19 patients is myocarditis,
and imaging findings included evidence of diffuse myocardial edema
and myocardial fibrosis. However, it is important to note that most
of the reported findings were mild increases in T1 and T2 signal
intensity, and the clinical significance of isolated T1/T2
abnormalities associated with COVID-19 still remains unknown
[26,27].
CARDIAC INVOLVEMENT AFTER RECOVERY FROM ACUTE COVID 19 DISEASE -
POST-ACUTE COVID 19 (PASC) or LONG COVID-19 SYNDROME
Certain patients infected with SARS-CoV-2 continue to have
symptoms for weeks to months after apparent recovery from the acute
phase of the disease. Early reports suggest that up to 10% of
patients with COVID-19 may experience "PROLONGED OR LONG COVID
SYNDROME" or POST-ACUTE COVID 19 (PASC). Symptoms of PASC vary
widely in variety, severity, and duration [16]. Preliminary studies
suggest that up to 30% of patients may report symptoms as late as 9
months after acute infection [28]. The most common symptoms include
fatigue, decreased functional capacity and exercise tolerance,
shortness of breath, sleep problems, and palpitations. Some patients
describe difficulty thinking clearly ("brain fog"), anxiety and/or
depression. The exact predictors, duration, extent of cardiac (or
other organ) involvement, and potential effects of different
treatments for PASC require extensive research, which has already
begun [16].
The potential for long-term cardiac sequelae of myocardial damage
associated with COVID-19 has been highlighted in CMR studies of
recovered patients with evidence of myocardial fibrosis or
myocarditis reported in a wide range of 9% to 78% of patients
recovered from acute COVID-19. Among 100 post-COVID-19 patients who
underwent CMR 2 to 3 months after diagnosis, Puntmann et al reported
cardiac involvement in 78% with evidence of ongoing inflammation in
60%. On the day of imaging, 71% had elevated hs-TnT. Cardiac
symptoms were common and included atypical chest pain (17%),
palpitations (20%), and dyspnea and fatigue (36%). Recovered
patients had lower left ventricular (LV) ejection fractions and
larger LV volumes compared with risk factor-matched controls. These
CMR findings of myocarditis and myocardial fibrosis raise concerns
about potential long-term cardiac consequences, including increased
risk of heart failure and arrhythmia based on previous experience
with myocarditis. The presence of late gadolinium accumulation (LGE)
subepicardially and medially in the left ventricular wall associated
with myocarditis often implies myocardial necrosis in addition to
myocardial edema and has previously been associated with adverse
outcomes in multiple CMR studies of non-Covid-related myocarditis
[27]. Post-acute sequelae of SARS-CoV-2 infection, often called
post-acute COVID-19 syndrome or long-lasting-LONG COVID-19, can
occur in patients who are slow to recover. Of 143 patients who were
treated as outpatients after infection with COVID-19, only 12.6%
were asymptomatic. (Carfe A) [28]. Symptoms included fatigue
(53.1%), dyspnea (43.4%), joint pain (27.3%), and chest pain
(21.7%); 44.1% reported deterioration in quality of life. Among
1,733 discharged patients with COVID-19 followed for an average of 6
months after symptom onset, the most common symptoms were fatigue or
muscle weakness (63%), difficulty sleeping (26%), and anxiety or
depression (23%). Greater disease severity during hospitalization
was associated with reduced pulmonary diffusion capacities and
abnormal chest radiography. (Huang C. 2021). The contribution of
cardiac changes after COVID and acute myocardial injury to the
symptoms of post-acute COVID-19 syndrome is unclear [29].
PROOF OF DIRECT VIRAL HEART INFECTION BY PATHOHISTOLOGY
Cardiac autopsies showed cardiomegaly, right ventricular
enlargement, lymphocytic myocarditis (14%–40%), focal pericarditis
(19%), endocardial thrombosis (14%), or endotheliitis and thrombosis
of small coronary vessels (19%). The cardiac tropism of SARS-CoV-2
was initially established by quantitative RT-PCR detection of viral
RNA in postmortem hearts of patients with COVID-19 and then in
endomyocardial biopsies of patients with suspected myocarditis. The
cardiac cellular tropism of SARS-CoV-2 has now been demonstrated by
in situ labeling of SARS-CoV-2 RNA and electron microscopic
detection of virus-like particles within cardiomyocytes,
interstitial cells, and cardiac endothelial cells post mortem
[30,31]. Autopsies in patients with acute myocarditis have recently
shown evidence of viral infection, and replication within
cardiomyocytes. The preponderance of evidence suggests that
SARS-CoV-2 can readily infect human cardiac myocytes and can be
detected in myocytes at autopsy or endomyocardial biopsy in patients
with and without clinical evidence of cardiac involvement. There are
pathohistological findings of clear myocarditis in individual cases
where all elements strongly suggest COVID-19 myocarditis or direct
cardiomyocyte damage in an extremely strong inflammatory reaction
(cytokine storm) caused by viremia rather than a microvascular
myocardial lesion [14,32]
Of 277 hearts in 22 autopsy studies of COVID-19, only 20 cases of
myocarditis (7.2%) were reported. In contrast to the low prevalence
of myocarditis, interstitial macrophage infiltration without
cardiomyocyte degeneration was common in a multicenter COVID-19
autopsy series (18 of 21 cases, 86%) [33]. Other more common
histologic findings reported in the COVID-19 autopsy series include
perivascular and inflammatory myocardial infiltrates, endocardial
and small vessel thrombosis, endotheliitis, and myocyte
degeneration. One study of 39 autopsied hearts detected SARS-CoV-2
by qRT-PCR in 24 (61.5%) cases, with 16 hearts showing high viral
loads (>1000 genomic copies per mg of total RNA) [34,35]. It remains
to be determined whether the heterogeneity of cardiac histopathology
in COVID-19 signifies different endophenotypes of the myocardial
lesion of COVID-19 or the continuity of a single pathological
process.[16] .
PROLONGED EXERCISE INTOLERANCE AND DYSAUTONOMY
There is increasing evidence of prolonged symptoms of COVID-19
after a period of acute infection (post-acute covid, long covid)
with prolonged exercise intolerance (failure to exert effort) which
is becoming a common finding not only in competitive athletes and
active individuals, but also in many young and elderly people
survivors of COVID-19 [3,16]. Common symptoms associated with
myocarditis and post-COVID syndrome include chest pain, dyspnea, and
palpitations. CMR findings of a cardiac lesion, small nerve fiber
neuropathy caused by the COVID-19 virus, and dysautonomia are likely
causes. Postural orthostatic tachycardia syndrome associated with
COVID-19 is common. The relative poor cardiac fitness during periods
of exercise and training limitations is often confounding in
situations when trying to delineate the cause of failure to exercise
[3,16].
The potential for increased risk of sudden cardiac death in post-COVID
fibrosis or myocardial inflammation is of concern to athletes or
active individuals returning to exercise. The wide range of LGE
prevalence after COVID-19 has led to controversy over the routine
practice versus targeted use of CMR. Risk stratification with
noninvasive biomarkers, ECG, or echocardiography may be insensitive
for detecting CMR abnormalities. Conversely, ECG changes considered
abnormal in non-athletes may represent normal variants in athletes.
According to the American College of Cardiology, Sports, and
Exercise, athletes who have recovered from COVID-19 can return to
sports based on biomarkers and noninvasive cardiac imaging,
including ECG and echocardiogram [3,16]. Athletes are advised to
limit exercise to 5 days a week, minimally at first with a gradual
increase in exercise intensity. Cardiovascular risk assessment is
recommended for mild symptoms lasting longer than 10 days; for
moderate or severe symptoms, including hospitalization, further
cardiac testing depends on symptoms and abnormal findings on
baseline testing. The uncertainty of long-term consequences and the
potential for long-term evolution into chronic myocardial disease,
cardiomyopathy, and other cardiovascular complications, including
heart failure, chronic sinus tachycardia, autonomic dysfunction, and
arrhythmias, await further definition. In addition, studies are
needed to determine whether therapeutic interventions to moderate
the inflammatory response can also limit the extent of intermediate-
to long-term myocardial injury associated with COVID-19. Evaluation
of post-acute COVID-19 syndrome (long-COVID-19) and recommendations
for long-term surveillance, monitoring, and return to exercise or
sport remain areas for further evaluation [3,16].
PRINCIPLES OF THE THERAPEUTIC APPROACH TO COVID-19 INFECTION
WITH A FOCUS ON THE CARDIOVASCULAR SYSTEM
The most important principles in the therapeutic approach to
COVID-19 patients [16]: A) optimal supportive measures and treatment
of complications; B) treatment of existing chronic cardiovascular
diseases and conditions developed as part of COVID-19 according to
the current guidelines of professional societies and associations
(ESC, AHA/ACC) including inhibitors of the
renin-angiotensin-aldosterone system [14]; C) in cases of cytokine
storm associated with the development of ARDS and myocarditis,
consider the introduction of immunomodulatory therapy; D) individual
risk stratification for development of KV complications in COVID-19
infection, prevention of these, early recognition and treatment [ 14
]. Treatment of COVID- 19 and complications associated with COVID-19
[16] continues to develop rapidly as more treatments complete
testing in randomized trials. Treatment in early phase includes
antiviral medicines and monoclonal antibodies against SARS - CoV- 2.
Antiviral medicines. Remdesivir is nucleoside analog which
inhibits RNA dependent RNA polymerase and is the only antiviral
medicine approved by US Food and Drug Administration (FDA) for
treatment of COVID-19 [16]. It is currently recommended to patients
hospitalized with moderate COVID-19 who need extra oxygen, but its
benefit has not been established in patients who require high flow
oxygen , non-invasive ventilation or mechanical ventilation .
Treatment lasts about 5 days, it can be prolongedto 10 days if there
are no clinical improvements [ 36 ] .
Monoclonal antibodies against SARS - CoV -2 which have been
approved by FDA for emergency use : Bamlanivimab plus etesevimab
(applied together) have been approved for treatment of mild to
moderate COVID- 19 in adults and pediatric outpatients [37].
Besides, FDA has issued permission for kasirivimab and imdevimab
applied together) for treatment of mild to moderate variant of
COVID-19 in adults and pediatric patients [38]. Potential
cardioprotective effects of treatment by anticytokines haven't been
determined yet due to inconsistencies in the results of clinical
trials[16].
Corticosteroids have showed benefit in a patient subgroup
with moderate COVID-19 whoneeded extra oxygen . In a randomized
evaluation trial of therapy for COVID-19, dexamethasone (6 mg one
time daily up to 10 days) reduced the 28 -day mortality, but
patients who didn’t need oxygen did not experience any benefits [16,
39]. In meta - analysis of 7 randomized controlled studies ( CT)
which included 1703 critically sick patients (including those who
needed mechanical ventilation ) with COVID-19, the use of systemic
dexamethasone , hydrocortisone or methylprednisolone resulted in the
reduction of risks of mortality from all causes by 34% after 28 days
[16,40].
"A "storm" of cytokine release", which comes from T cell
activation imbalance with unregulated interleukin release (IL)-6, IL
-17 and other cytokines , can contribute to CVD in COVID- 19.
Anti-IL-6 antibody therapy trial is ongoing. Activation of the
immune system together with the changes in immunometabolism can lead
to the instability of atherosclerotic plaques, contributing to the
development of acute coronary events [16].
The role of anticoagulation in COVID- 19. Many observational
or smaller studies have investigated which patients with COVID- 19
could benefit from anticoagulants or antiaggregation therapy, in
which dose and in which phase of the disease with different results
. While waiting for sufficiently strong, properly designed and
performed blinded randomized trials, many institutions have adopted
the prophylaxis of escalated doses in all or specific _ groups of
hospitalized patients with COVID-19. Documents about consensus
generally recommend tracking the available medical recommendations
based on the evidence in order to avoid a widespread use higher than
the prophylactic dose of anticoagulants , except if it is not used
as a part of a research study [16,41]. In general , risk from venous
thromboembolism (VTE) in hospitalized patients reached its climax in
the early stage of the pandemic, but later the incidence decreased
thanks to the adoption of prophylactic anticoagulation. A big study
of Danish registers based on the national population suggests that
the risk from the VTE in hospitalized patients with COVID -19 is low
to moderate and that it's not significantly higher than the risks
from the VTE in hospitalized SARS - CoV -2- negative patients and
patients with flu [42]. VTE Risk in the period after dismissal and
in ambulatory cases of COVID - 19 can be slightly elevated, but it
is much smaller than the risks in acutely ill and hospitalized
patients.
Antagonists of the renin-angiotensin-aldosterone system (RAAS
antagonists)
Following the discovery that SARS-CoV-2 uses ACE2 to enter the host
cell, concerns have been raised about the potential for ACE
inhibitors and ARBs to cause a compensatory increase in ACE2
expression and worsen prognosis among those with COVID-19.
Observational studies evaluating outcomes associated with the use of
ACE inhibitors and ARBs among patients with confirmed COVID-19
[43,44] and RCTs comparing continuation or withdrawal of these
agents among those hospitalized with COVID-19 have shown no adverse
effects on survival and other clinical outcomes [45,46] . Therefore,
continuation of ACE inhibitors and ARBs during the course of
COVID-19 disease is recommended for patients treated with these
drugs. It also appears that in experimental models, ARBs may have a
potentially protective effect. A recent observational study of over
8910 patients from 169 hospitals in Asia, Europe, and North America
showed no adverse association of ACEIs or ARBs with in-hospital
mortality, while a study in Wuhan showed that in 1128 hospitalized
patients, ACEI/ARB use was associated with a lower risk from
infection with COVID-19 or serious complications or death from
infection with COVID-19. This is consistent with previous guidelines
from the major cardiovascular associations, which state that
patients on ACEIs or ARBs should not discontinue these medications
[16].
ORGANIZATION OF CARE AND SPECIFICITY OF THE MOST IMPORTANT CVD
DURING THE COVID-19 PANDEMIC
Non-ST elevation acute coronary syndromes (NSTEMI)
Management of patients with NSTE ACS should be guided by risk
stratification [3]. Testing for SARS-CoV-2 should be performed as
soon as possible after the first medical contact, regardless of the
treatment strategy, so that the healthcare professional can
implement adequate protective measures and care pathways. Patients
should be categorized into 4 risk groups (ie, very high risk, high
risk, intermediate risk, and low risk) and managed accordingly.
Patients with an increase in troponin and without acute clinical
signs of instability (ECG changes, recurrence of pain,
hemodynamically stable) can be treated with a primarily conservative
approach. Non-invasive imaging with CCTA can speed up risk
stratification, avoid an invasive approach and allow early
discharge. For high-risk patients, the medical strategy aims at
stabilization while planning an early (< 24 hours) invasive
strategy. In the case of a positive SARSCoV-2 test, patients should
be transferred for invasive treatment to a COVID-19 hospital
equipped to treat the patients positive for COVID-19.
Intermediate-risk patients should be carefully evaluated considering
alternative diagnoses of T1MI, such as type II MI, myocarditis or
myocardial lesion due to respiratory distress or multiorgan failure,
or Takotsubo. In case any of the differential diagnoses seems
plausible, a non-invasive strategy should be considered and CT scan
coronary angiography (CCTA) should be preferred [3].
ST segment elevation myocardial infarction (STEMI)
The COVID-19 pandemic should not compromise timely reperfusion via
percutaneous balloon angioplasty with stent placement (PCI) or
thrombolytic therapy in patients with STEMI [3] .
According to current guidelines, reperfusion therapy remains
indicated in patients with symptoms of ischemia lasting less than 12
hours with permanent ST-segment elevation on ECG in at least two
adjacent leads. At the same time, there must be safety for
healthcare workers and in the absence of testing for SARS-CoV-2, all
patients should be treated as if they were Covid-19 positive. The
safety of healthcare professionals is of utmost importance to avoid
healthcare worker infections and further spread of infection.
Chronic coronary syndromes (CCS)
Patients with Chronic Coronary Syndrome (CCS) with a clinical
scenario of stable angina pectoris are generally at low risk of CV
events, which allows delaying diagnostic and/or interventional
procedures in most cases [3] .
Medical therapy should be optimized and/or intensified depending on
the clinical status. Clinical monitoring of a patient via
telemedicine is justified for the early detection of unstable angina
or changes in clinical status that may require hospital admission in
high-risk patients.
Acute heart failure (AHF)
Bilateral COVID-19 pneumonia often leads to worsening hemodynamic
status due to hypoxemia, dehydration, and hypoperfusion. The main
mechanisms of AHF in COVID-19 are acute myocardial ischemia,
myocardial infarction or inflammation (myocarditis), acute
respiratory distress syndrome (ARDS), acute kidney damage and
hypervolemia, stress-induced cardiomyopathy, and tachyarrhythmias
[3] .
Clinical presentation, the presence of existing CV comorbidities and
the findings of X-ray thorax (cardiomegaly and/or bilateral pleural
effusion, congestion of the lung wings at the bases) are of utmost
importance. Significantly elevated levels of BNP and not NT-proBNP
also suggest acute HF. Careful use of point-of-care (POC)
transthoracic echocardiography (TTE) is recommended to prevent
contamination of personnel and/or equipment from the patient. The
same treatment strategy for acute HF can be applied in patients with
and without COVID-19 [3,47]. Regarding prognosis, in a recent report
23% of all hospitalized patients developed AHF, while the prevalence
of HF was significantly higher in fatal cases compared with
survivors (52% vs. 12%, P < 0.0001). [3].
Chronic heart failure (CHF)
The risk of infection with COVID-19 may be higher in chronic heart
failure HF patients due to age and the presence of multiple
comorbidities. In ambulatory stable patients with HF, without urgent
cardiac conditions, the prescribing physician should refrain from
hospital treatment. Medical therapy according to the guidelines
(including the five parallel pillars of therapy according to the new
ESC guideline [3,47] Beta-blockers, SGLPT-2 inhibitors,
mineralocorticoid receptor antagonists (MRA), loop of Henle
diuretics for congestion and one of the RAAS inhibitors, preferably
sacubitril/valsartan or ACEI, OR ARBa), should be continued in
patients with chronic HF, regardless of COVID-19. The implementation
of telemedicine to provide medical advice and follow-up of stable
patients with COVID-19 is important.
Arterial Hypertension
An association between hypertension and risk of severe complications
or death from COVID-19 infection was found, with a confounding lack
of effect of age and comorbidities associated with aging and
hypertension. However, there is currently no evidence to suggest
that hypertension per se is an independent risk factor for severe
complications or death from COVID-19 infection [3]. Despite much
speculation, evidence from a recently published series of
observational cohort studies suggests that previous or current
treatment with an ACEI or ARB does not increase the risk of
infection with COVID-19 or the risk of developing severe
complications from infection with COVID-19 compared to the risk in
patients taking other antihypertensive drugs. Treatment of
hypertension should follow the existing recommendations in the ESC-ESH
Guidelines. No changes to these treatment recommendations are
necessary during the COVID-19 pandemic [3] .
COVID 19 Myocarditis
Limited clinical experience indicates that SARS-CoV-2 can lead to
all forms of myocarditis from subclinical to fulminant myocarditis.
Myocarditis should be suspected in patients with COVID-19 and acute
chest pain, ST segment changes, cardiac arrhythmia, and hemodynamic
instability. In addition, dilatation of the left ventricle (LV) with
reduced ejection fraction (EF), global or multisegmental
hypocontractility of the LV with a significant increase in
cardiotroponin T and I and the level of both or only one natriuretic
peptide (BNP I / or NTproBNP) with the exclusion of significant
chronic coronary disease are elements for establishing a working
clinical diagnosis. In particular, myocarditis should be suspected
in COVID-19 patients with acute heart failure: pulmonary edema or
cardiogenic shock and without anamnestic data on previous CV
disease. Echocardiography, as the first and routine imaging method,
often shows diastolic dysfunction, multisegmental hypocontractility,
dilatation of both ventricles and a significant decrease in systolic
function - a drop in LV ejection fraction (LVEF) and sometimes a
small pericardial effusion. Advanced echocardiographic methods, such
as myocardial deformation imaging (strain-strain imaging) Myocardial
damage due to SARS-CoV-2-infection, specific deformation (strain)
abnormalities in the basal segments of the left ventricle were
highly prevalent even in patients with mild symptoms [17]. MSCT of
the coronary arteries (CCTA) is suggested as the best approach to
rule out concomitant coronary disease and cardiomagnetic resonance (CMR),
if available, can be used for further diagnostic evaluation.
Endomyocardial biopsy is not recommended in patients with COVID-19
with suspected myocarditis [3].
Efficacy of anticovid vaccination and post-vaccination
myocarditis
Vaccines have shown efficacy in reducing morbidity and mortality
from COVID-19 in randomized clinical trials and real-world studies,
which also reduce cardiovascular complications. Their widespread use
has led to a significant reduction in the incidence of COVID-19.
As of July 2021, the CDC's Adverse Event Reporting System (VAERS)
has received over 1,100 reports of myocarditis or pericarditis after
receiving a COVID-19 vaccination (primarily mRNA vaccine) and
confirmed about 70% of them. In Europe (EEA), cases of myocarditis
have also been reported with mRNA vaccines and with AstraZeneca
vaccine, mostly in young adults, more often in men and usually after
the second dose of the vaccine. Myocarditis, which can be detected
by cardiac magnetic resonance imaging, usually occurs within 3 to 5
days after vaccination and presents with chest discomfort, an
abnormal EKG, and elevated troponin. Although the exact mechanism is
unknown, it is probably immunologically mediated. The possible
incidence of asymptomatic cases, risk factors and long-term effects
remain to be determined. Overall, myocarditis following COVID-19
immunization appears to be rare (∼24 doses per million vaccines),
often mild, and probably self-limiting in most cases. Treatment is
primarily supportive [48,49].
CONCLUSION
Acute cardiac lesion is a common extrapulmonary manifestation of
COVID-19 with potential chronic consequences. Clinical
manifestations include direct cardiac damage and indirect immune
response mechanisms that affect the cardiovascular system and have
implications for the treatment of patients after recovery from acute
COVID-19 infection. Hypertension (56.6%) and diabetes (33.8%) are
the most common comorbidities in those infected with COVID-19,
requiring hospitalization.
Cardiovascular manifestations of COVID-19 vary, and acute infection
is associated with a wide range of cardiovascular complications,
including acute coronary syndromes, stroke, acute-onset heart
failure, arrhythmias, myocarditis, venous thromboembolism, and
cardiac arrest.
The most common direct damage to the heart is an acute heart lesion,
defined by a significant increase in cardiac troponins in the serum
in >12% of infected and echocardiographic signs of damage to the
texture of the myocardium due to inflammation, impairment of
segmental mobility, global systolic and diastolic function of the
left ventricle and inflammation of the pericardium. Among
hospitalized patients with COVID- 19, the evidence about acute
damage of heart function is common: acute heart insufficiency
(3%-33%), cardiogenic shock (9%-17%), ischemia or myocardial
infarction (0.9%–11%), ventricular dysfunction (left ventricular
[10%–41%], right ventricular [33%–47%], biventricular [3%–15%]),
stress cardiomyopathy (2%–5.6%), arrhythmias (9%–17%), venous
thromboembolism (23%–27%).
Elevated troponin T is associated with more frequent development of
severe complications: adult respiratory distress syndrome (ARDS),
malignant arrhythmias (VT, VF), acute coagulopathy and acute kidney
damage. Numerous individual cases indicate extremely high values and
dynamics of troponin T typical for non-occlusive myocardial
infarction with normal coronary arteries. Pathohistological findings
of myocarditis strongly suggest COVID-19 myocarditis or direct
damage to cardiomyocytes in an extremely strong inflammatory
reaction, a cytokine storm, caused by viremia.
About 10% of patients with COVID-19 may experience "LONG COVID
SYNDROME" or POST-ACUTE COVID 19 (PASC). The symptoms of PASC vary
widely in variety, severity, and duration.
Theoretically, the predicted increases in Angiotensin II levels by
COVID-19 infection can be curbed by administration of maximal doses
of ACE inhibitors and AT1 receptor blockers.
Cardiovascular dysfunction and disease are often fatal complications
of severe infection with the COVID-19 virus, and cardiac
complications can occur, even in patients without underlying heart
disease, as part of an acute infection and are associated with a
more severe form of COVID-19 disease and increased mortality.
LITERATURE:
- Rossouw TM, Anderson R, Manga P and Feldman C. Emerging Role
of Platelet-Endothelium Interactions in the Pathogenesis of
Severe SARS-CoV-2Infection-Associated Myocardial Injury. Front
Immunol. 2022;13:776861. doi: 10.3389/fimmu.2022.776861. PMCID:
PMC8854752 PMID: 35185878
- AlShahrani I, Hosmani J, Shankar VG, AlShahrani A, Togoo RA,
et al. COVID-19 and cardiovascular system-a comprehensive
review. Rev Cardiovasc Med. 2021;22(2):343-351. doi:
10.31083/j.rcm2202041.
- The European Society for Cardiology. ESC Guidance for the
Diagnosis and Management of CV Disease during the COVID-19
Pandemic.
https://www.escardio.org/Education/COVID-19-and-Cardiology/ESCCOVID-19-Guidance.
(Last update: 10 June 2020). Dostupno na:
https://www.medbox.org/document/esc-guidance-for-the-diagnosis-and-management-of-cv-disease-during-the-covid-19-pandemic#GO
- Mina K. Chung , Joseph Loscalzo et al. COVID-19 and
Cardiovascular Disease. Circulation Research.
2021;128:1214–1236. DOI: 10.1161/CIRCRESAHA.121.317997 April 16,
2021 1219.
- Hosseiny M. et al. Radiology perspective of coronavirus
disease 2019 (COVID-19) lessons from severe acute respiratory
syndrome and Middle East Respiratory Syndrome. AJR Am J
Roentgenol. 2020; 5: 1-5.
- Azevedo RB et al. Covid-19 and the cardiovascular system: a
comprehensive review. Journal of Human Hypertension
2021;35(1):4–11. https://doi.org/10.1038/s41371-020-0387-4
- Isabela da Silva Costa et al. The Heart and COVID-19: What
Cardiologists Need to Know Arq Bras Cardiol. 2020;
114(5):805-816.
- Wu Z, et al. Characteristics of and important lessons from
the coronavirus disease 2019 (COVID-19) outbreak in China:
summary of a report of 72314 cases from the Chinese Center for
Disease Control and Prevention. JAMA. 2020;323(13):1239-1242.
- Wang D, et al. Clinical characteristics of 138 hospitalized
patients with 2019 novel coronavirus-infected pneumonia in
Wuhan, China. JAMA. 2020;323(11):1061-1069.
- Jing Yang et al, Prevalence of comorbidities and its effects
in patients infected with SARS-CoV-2: meta-analysis. Int J
Infect Dis. 2020; 94: 91–95.
- Guo T. et al. Cardiovascular implications of Fatal Outcomes
of Patients with Coronavirus Disease 2019 (COVID-19). JAAC,
(published online March 27), 2020;5(7):811-818.
- Shi S, et al. Association of cardiac injury with mortality
in hospitalized patients with COVID-19 in Wuhan, China. JAMA
Cardiol. 2020;5(7):802-810.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y,
Song B, Gu X, et al. Clinical course and risk factors for
mortality of adult inpatients with COVID- 19 in Wuhan, China: a
retrospective cohort study. Lancet. 2020;395:1054–1062. doi:
10.1016/S0140-6736(20)30566-3.
- Madjid M. et al. Potential Effects of Coronaviruses on the
Cardiovascular System. JAMA Cardiology. 2020;5(7):831-840.
- Austin Tutor et al. Spectrum of Suspected Cardiomyopathy Due
to COVID-19: A Case Series. Curr Probl Cardiol 2021;46:100926.
- Libby P, Bonow OR, Douglas L, Mann DL, Tomaselli FG, et al.
BRAUNWALD’S HEART DISEASE: A TEXTBOOK OF CARDIOVASCULAR
MEDICINE, TWELFTH EDITION. ELSERVIER 2022;1743-63.
- Stephan Stöbe et al. Echocardiographic characteristics of
patients with SARS CoV 2 infection. Clinical Research in
Cardiology 2020;109(12):1549-1566.
- COVID-19 and Cardiology Last updated on 10 February 2022.
Dostupno na
https://www.escardio.org/Education/COVID-19-and-Cardiology
- Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac
manifestations in COVID- 19: a systematic echocardiographic
study. Circulation. 2020;142(4):342–353.
- Jajodia A, Ebner L, Heidinger B, K CA, Prosch H. Imaging in
corona virus disease 2019 (COVID-19)-A scoping review. Eur J
Radiol Open 2020;7:100237.
- Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP,
Raoof S et al. The Role of chest imaging in patient management
during the COVID-19 pandemic: A multinational consensus
statement from the Fleischner Society. Radiology 2020;
296(1):172-80.
- Giustino G et al. Characterization of myocardial injury in
patients With COVID-19. J Am Coll Cardiol. 2020;76:2043–2055.
- Ogungbe O, Kumbe B, Fadodun OA, Latha T, Meyer D et. al.
Subclinical myocardial injury, coagulopathy, and inflammation in
COVID-19: A meta-analysis of 41,013 hospitalized patients. IJC
Heart & Vasculature. 2022;40:100950.
doi.org/10.1016/j.ijcha.2021.100950
- Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden
AC, et. al. COVID-19-Associated Nonocclusive Fibrin Microthrombi
in the Heart. Circulation. 2021;143(3):230-243. doi:
10.1161/CIRCULATIONAHA.120.050754.
- Bernhard Metzler, Ivan Lechner, [...], and Sebastian J.
Reinstadler. Cardiac injury after COVID-19: Primary cardiac and
primary non-cardiac etiology makes adifference. Int J Cardiol.
2022; 350: 17–18.
- Ojha V et al, Cardiac magnetic resonance imaging in
coronavirus disease 2019 (COVID-19): a systematic review of
cardiac magnetic resonance imaging findings in 199 patients. J
Thorac Imaging. 2020;36:73–83.
- Puntmann VO, et al. Outcomes ofcardiovascular magnetic
resonance imaging in patients recently recovered from
Coronavirus Disease 2019 (COVID-19). JAMA Cardiol.
2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557.
- Carfě A, Bernabei R, Landi F; Gemelli Against COVID-19
Post-Acute Care Study Group. Persistent symptoms in patients
after acute COVID-19. JAMA. 2020;324:603–605. doi:
10.1001/jama.2020.12603.
- Huang C, et al. 6-month consequences of COVID-19 in patients
discharged from hospital: a cohort study. Lancet.
2021;397:220–232.
- Buja LM, Wolf DA, Zhao B, et al. The emerging spectrum of
cardiopulmonary pathology of the coronavirus disease 2019
(COVID- 19): report of 3 autopsies from Houston, Texas, and
review of autopsy findings from other United States cities.
Cardiovasc Pathol. 2020;48:107233.
- Roshdy A, Zaher S, Fayed H, Coghlan JG. COVID- 19 and the
heart: a systematic review of cardiac autopsies. Front
Cardiovasc Med. 2021;7:626975.
- Cardiology in the Time of COVID-19: Current Status of the
COVID-19 Pandemic. dostupno na:
https://www.youtube.com/watch?v=KeLcqsISrZg
- Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-
19 autopsies: cardiovascular findings across 277 postmortem
examinations. Cardiovasc Pathol. 2021;50:107300.
- Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral
SARS- CoV- 2 genomes and histopathological changes in
endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440–2447.
- Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson
JL. Harrison's Principles of Internal Medicine, Twenty-First
Edition (Vol.1 & Vol.2) 21st Edition. McGrawHill 2022; 1508-11.
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10
Days in patients with severe Covid- 19. New Engl J Med.
2020;383(19):1827–1837.
- Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab
as monotherapy or in combination with etesevimab on viral load
in patients with mild to moderate COVID- 19: a randomized
clinical trial. J Am Med Assoc. 2021;325(7):632–644.
- Weinreich DM, Sivapalasingam S, Norton T, et al. REGN- COV2,
a neutralizing antibody cocktail, in outpatients with Covid- 19.
New Engl J Med. 2020;384(3):238–251.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al.
Dexamethasone in hospitalized patients with Covid- 19. New Engl
J Med. 2021;384(8):693–704.
- Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy
for critically ill patients with COVID- 19: a structured summary
of a study protocol for a prospective meta- analysis of
randomized trials. Tri-als. 2020;21(1):734.
- Moores LK, Tritschler T, Brosnahan S, et al. Prevention,
diagnosis, and treatment of VTE in patients with coronavirus
disease 2019: CHEST guideline and expert panel report. Chest.
2020;158(3):1143–1163.
- Dalager- Pedersen M, Lund LC, Mariager T, et al. Venous
thromboembolism and major bleeding in patients with COVID- 19: a
nationwide population- based cohort study. [published online
ahead of print January 5, 2021]. Clin Infect Dis.
https://doi.org/10.1093/cid/ciab003
- Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-
angiotensin- aldosterone system inhibitors and risk of Covid-
19. New Engl J Med. 2020;382(25):2441–2448.
- Mancia G, Rea F, Ludergnani M, et al. Renin- angiotensin-
aldosterone system blockers and the risk of Covid- 19. New Engl
J Med. 2020;382(25):2431–2440.
- Lopes RD, Macedo AVS, de Barros ESPGM, et al. Effect of
discontinuing vs continuing angiotensin- converting enzyme
inhibitors and angiotensin II receptor blockers on days alive
and out of the hospital in patients admitted with COVID- 19: a
randomized clinical trial. J Am Med Assoc. 2021;325(3):254–264.
- Cohen JB, Hanff TC, William P, et al. Continuation versus
discontinuation of renin- angiotensin system inhibitors in
patients admitted to hospital with COVID- 19: a prospective,
randomised, open- label trial. Lancet Respir Med.
2021;9(3):275–284.
- Bastać D, Joksimović Z, Pavlović S, Bastać M, Raščanin A,
Đorđioski I. PROMENA PARADIGME U LEČENJU HRONIČNE SRČANE
INSUFICIJENCIJE PO ESC VODIČU 2021 -NOVI INOVATIVNI LEKOVI U
FOKUSU. TMG 2022; 47(1):40-47.
- Diaz GA, Parsons GT, Gering SK, et al. Myocarditis and
pericarditis after vaccination for COVID-19. JAMA. 2021 Aug
4:e2113443.
- Montgomery J, Ryan M, Engler R, et al. Myocarditis following
immunization with mRNA COVID-19 vaccines in members of the US
military. JAMA Cardiol. Published online June 29, 2021.
https://doi.org/10.1001/jamacardio.2021.2833.
|
|
|
|


