| |
|
|
INTRODUCTION
Subclinical hypothyroidism (SKH) is a common clinical condition
about which there is much controversy. To date, there has been no
definite consensus among thyroidologists on several aspects. First
of all, the question arises whether it is necessary to do screening
at SKH, ie. actively search for disorder in a wider asymptomatic
population at routine periodic / preventive examinations, or find
cases according to clinical indications. Another aspect of the
problem is how to assess the significance of this clinical
condition, as well as possible adverse effects on the cardiovascular
system, metabolic parameters and mental health of the individual
patient. From the first two questions the third one arises, and that
is what kind of therapeutic approach to have in SKH - to treat it or
not.
WHAT IS SUBCLINICAL HYPOTHYROIDISM
Subclinical hypothyroidism is a thyroid disorder in which the
level of thyroid hormones (TH), thyroskin (T4) and triiodothyronine
(T3) in the blood is normal, but the level of thyrotropin (TSH), a
pituitary hormone that regulates thyroid function, is elevated. This
is a biochemical diagnosis, because patients are typically
asymptomatic and without signs of disease and the detection of SCH
is usually accidental. Over time, SKH may progress to clinical
hypothyroidism (KH). [1,2] SKH, depending on the duration and degree
of TSH elevation, may be associated with an increased risk of
cardiovascular (CV) disease and CV mortality, adverse effects on
metabolic parameters, cognitive dysfunction, anxiety and depression
[2,3]. Several alternative names describing the condition of SKH
have been suggested such as: compensated hypothyroidism, preclinical
hypothyroidism, mild hypothyroidism, decreased thyroid reserve, mild
thyroid weakness [4].
WHAT IS THE PREVALENCE OF SUBCLINICAL HYPOTHYROIDISM
The estimated total prevalence of SKH in the general population
is 4-10% depending on the characteristics of the examined
population, ie. gender, age, race, geographical area, iodine status
[4]. SKH is more common in women and the elderly. In women, the
prevalence is 8-10%, and in women older than 60, the published
prevalence is up to 20% [5,6]. The prevalence is about three times
higher in whites than in blacks [7]. Also, during an increase in
iodine intake in a previously iodine-deficient population, there may
be a slight increase in the prevalence of SKH and thyroid
autoimmunity [8]. There are studies in which the prevalence of SKH
in people with metabolic syndrome (MetS) is almost two and a half
times higher [9]. In addition, SKH is more common in patients with
Type 2 Diabetes Mellitus (DM T2) than in the healthy population and
is about 10% according to some reports [10]. SKH is a relatively
common condition in patients with chronic renal failure (HBI) and
can be found in about 18% of patients with HBI who are not on
dialysis [11]. The reported incidence of SCH in pregnant women is
2-2.5%, in some countries such as China, Belgium and northern Spain
even 4-13.7%, and in children the prevalence is less than 2% [12].
Of course, in order to assess the prevalence of this condition in
the population / populations, accurate registration and adequate
health statistics are necessary. Estimated prevalences are often
based on meta-analyzes of published articles in available databases
of professional and scientific papers, in which data from limited
samples of respondents are analyzed. However, differences in the
estimated prevalence may also be influenced by different diagnostic
criteria for this condition, e.g. use, or not, of specific serum TSH
reference ranges, in this case upper limits of the reference range
for individual population groups. Research shows that it is
necessary to determine the distribution of concentration and range
of normal TSH values, probably due to genetic factors, according to
age and race, or other specific characteristics of the population,
which would be used to assess the presence of thyroid dysfunction
(TD) [13]. In this regard, some authors believe that the prevalence
of SKH in the elderly is overestimated, because the upper limit of
the reference range for TSH increases with age [14].
CAUSES OF SUBCLINICAL HYPOTHYROIDISM
The most common cause of subclinical hypothyroidism, as well as
clinical, in areas with sufficient iodine intake, is chronic
autoimmune thyroiditis - Hashimoto's thyroiditis (HT), atrophic
thyroiditis (AT), postpartum thyroiditis (PPT) [3]. Autoimmune
thyroid diseases (AITB), which include HT, AT and PPT, are 5 to 10
times more common in women than in men, the prevalence increases
with age, they are more common in people with other autoimmune
diseases, as well as in their blood relatives [3, 15-17].
AITB is characterized by pathological infiltration of the thyroid
gland by sensitized T lymphocytes and the presence of thyroid
autoantibodies in the blood - antimicrosomal antibodies / antibodies
to thyroid peroxidase (TPOAb), antithyroglobulin antibodies (TgAb),
prescription (TgAb) and 3 antibodies, [18], TSA [19], TSA
antibodies. Determination of these antibodies in serum is one of the
key diagnostic methods for the diagnosis of AITB.
On the other hand, a very common cause of SCC is iodine deficiency
in the diet, because the problem of iodine deficiency areas is still
pronounced worldwide [20]. Iodine is a microelement necessary for
the production of thyroid hormones (TH), thyroxine (T4) and
triiodothyronine (T3), which must be taken into the body through
food, at least 150 µg per day.
Causes of SKH can also be iatrogenic, for example the condition
after radioiodine, or surgical therapy of benign and malignant
diseases of the thyroid gland, ie. diffuse toxic goiter, toxic
adenoma, polynodose toxic goiter, benign and malignant atoxic
nodular goiter. Also, radiation therapy to the thyroid gland can
lead to radiation therapy of the neck due to non-thyroid diseases of
the head and neck, including lymphoma.
Iatrogenic SKH can also be pharmacological, caused by the use of
drugs for non-thyroid diseases, or diagnostics, such as iodine-rich
antiarrhythmics, amiodarone, then lithium, used in psychiatry,
iodine contrast agents, interferon alpha and other cytokines,
tyrosine kinase inhibitors (TKI), antituberculotic
Paraaminosalicylic acid (pAS), less often aminoglutethimide, which
lead to SKH by various mechanisms e.g. thyroid cytotoxicity,
blockade of TH production and release of excess iodine, reducing
blood supply to thyroid tissue, action on type 2 and 3 deiodinases,
which participate in the production of TH and their metabolites, and
others [21-26]. Of course, there are also antithyroid drugs that are
given in the treatment of hyperthyroidism, ie. methimazole and
propyl thyrouracil, may lead to SKH.
Infiltrative diseases, such as amyloidosis, sarcoidosis,
hemochromatosis, scleroderma, cystinosis, Riddle's thyroiditis, can
also affect the thyroid gland and be the cause of reduced functional
reserves, ie. SKH [27,28].
As already mentioned, SKH as a consequence of AITB can often be
associated with other autoimmune diseases, e.g. DM type 1, Addison's
disease, rheumatoid arthritis [29-31], but also chromosomal
disorders such as Down's or Turner's syndrome [32,33], which
requires mandatory examination of thyroid function in patients with
these diseases and syndromes.
Consumptive, or "expendable" SKH is a rare condition that occurs in
patients with hemangiomas and other tumors in which type 3
deiodinase is expressed, causing accelerated degradation of T4 and
T3 [34].
Finally, transient SCH can be found in patients in the recovery
phase from non-autoimmune thyroiditis, subacute and painless
thyroiditis, as well as during recovery from severe non-thyroid
disease (NTB) [35].
THE COURSE OF SUBCLINICAL HYPOTHYROIDISM
In most patients, SKH remains stable over time. Depending on the
degree of increase in the initial level of TSH, annually 5-8% of
patients with SKH have a progression to clinical hypothyroidism (KH)
[36]. On the other hand, thyroid function may return to normal over
time in 6-35% of patients, also depending on baseline TSH levels as
well as thyroid autoantibody levels [37]. In patients with elevated
TPOAb, the progression of SKH to KH is 4.3% per year, and in those
with normal TPOAb levels, almost twice as low, 2.6% per year [38].
Therefore, after the diagnosis of SCH, thyroid function tests (TFT)
are repeated in 8-12 weeks and additional measurement of thyroid
autoantibody levels is performed. If SKH persists, TFTs are repeated
for 6 months during the first two years of follow-up, and then once
a year if the findings are stable. In contrast, if TFTs are normal
after repeated determinations and the patient has no symptoms,
goiter, and elevated thyroid autoantibodies, further monitoring is
not necessary [3].
DIAGNOSIS OF SUBCLINICAL HYPOTHYROIDISM
The diagnosis of SKH is made when elevated TSH values are
detected in the patient (the reference range of most tests is 0.4 -
4.0 to 5 m IU/L) with normal FT4 values in the blood [39] . Bearing
in mind that the diagnosis of SKH is based on the results of
laboratory analyses, the specificity, sensitivity and reference
values of the applied test should be taken into account, and the
finding should be interpreted accordingly [40]. Although elevated
serum TSH is most often a sign of primary hypothyroidism, it is
important to know that measured concentrations may be elevated
(usually <8 mU/L) in individuals over 65 years of age without
clinical and laboratory evidence of thyroid disease [41]. Other
conditions, such as post-radiotherapy of the neck, adrenal
insufficiency, pregnancy, use of certain drugs (lithium, AMD), or
the presence of specific antibodies in the blood (HAMA, or macro TSH)
may mimic SKH [42-44]. In addition, pathological obesity due to the
effect of leptin on thyrotropin releasing hormone (TRH) leads to a
reversible increase in blood TSH [45]. Fluctuations in TSH
concentration are expected in acute, especially severe netiroid
diseases, as well as after surgical procedures - hemithyroidectomy,
which should be taken into account when interpreting laboratory
findings [42,46]. Laboratory diagnosis should be postponed for 2-3
months after recovery from acute diseases, due to the effects of
cytokines on TSH concentration, and supplementation with biotin,
which is a part of many multivitamins (especially those recommended
for hair and nail health) should be stopped at least 2 days before
laboratory tests, Analysis, due to interference with immunoassays
[42,47,48].
There are two categories of SKH according to the degree of TSH
increase. Slightly elevated TSH, of 4–10 m IU/L, found in 80–90% of
patients, and significantly elevated TSH,> 10 m IU/L [3]. After the
diagnosis of TSH, the cause should be determined, ie. an etiological
diagnosis should be made. Additional laboratory analyses in order to
establish the etiological diagnosis are measurement of thyroid
autoantibodies (TAT), TPOAb mainly due to higher sensitivity and
less often TgAb, as well as ultrasound examination of the thyroid
gland which can detect characteristic parenchymal changes in
autoimmune thyroiditis, which is the most common cause of SKH [50].
The level of TSH in a healthy person has small variations over time,
about 1/3 of the reference range, which is called its own "TSH
setpoint", which tends to increase with age [51,52]. Thus, as
mentioned, in the elderly we use a wider reference range (4.0-7.0 m
IU/L), i.e., a slightly elevated TSH level in the elderly is
considered a physiological adaptation to aging [41].
In both healthy and SKH, TSH levels have circadian fluctuations in
serum concentrations - the lowest concentration is in the early
afternoon, with about 30% higher concentrations in the evening and
overnight.
Delayed night peak TSH can be found in: night shift workers; those
with sleep disorders; after strenuous physical activity; in mood
disorders - depression [3].
Biologically inactive forms of TSH may be the reason for measured
higher TSH values in some individuals [53].
Let us repeat that the level of TSH correlates with BMI and markers
of insulin resistance, so the finding of TSH> 3.5 is common in obese
[54].
CLINICAL CHARACTERISTICS OF SUBCLINICAL HYPOTHYROIDISM
Symptoms
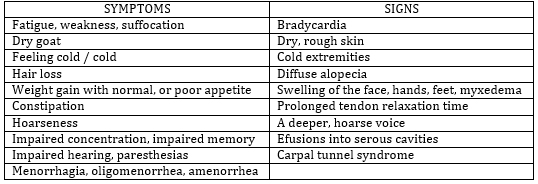
By definition, SKH is an asymptomatic condition, with no clinical
signs of hypothyroidism (Table 1). However, is SKH really
asymptomatic? Some studies show that a small but statistically
significant number of patients with SKH have more frequent symptoms
of hypothyroidism than healthy ones: drier skin, poorer memory,
slower thinking, weaker muscles, faster fatigue, more frequent
muscle cramps, greater winter fever, deeper and hoarse voice,
swollen eyes and more frequent constipation [5]. On the other hand,
since the symptoms and signs of hypothyroidism are general and can
occur in other conditions, some studies show that there is no
improvement in symptoms in patients with SCI when levothyroxine
substitution is introduced [55]. However, most patients with SCH do
not have hypothyroid symptoms.
Mood and mental health disorders
Based on many studies, it seems that there may be mild disorders of
declarative memory (knowledge of facts), procedural memory (skills
that are performed automatically) and mood in younger people with
SCC, which are improved by levothyroxine substitution [56]. However,
such evidence is generally not found in the population over 65 years
of age [57].
Table 1. Symptoms and signs of hypothyroidism
Obesity, glycoregulation, insulin resistance, diabetes
mellitus, dyslipidemia
Serum TSH levels are positively correlated with body weight [58] and
it has been shown that for each unit of increase in log TSH, body
weight is 2.3 kg higher in women and 1.1 kg in men [59]. In
contrast, a significant decrease in body weight is associated with a
decrease in TSH levels [60]. However, a sample relationship between
SKH and obesity has not been shown.
SKH could reduce insulin sensitivity by reducing the number of
glucose transporters in plasma membranes (cell organelle membranes)
and by directly affecting insulin secretion and clearance, as is
known to occur to a significant extent in hypothyroidism [61]. In
patients with established diabetes mellitus (DM) type 2, a change in
glycemic control may indicate SKH and long-term thyroid disorders,
while the prevalence of SKH with elevated TAT in a patient with type
1 DM is up to 30% [62].
Large epidemiological studies have shown a positive correlation
between TSH levels and dyslipidemia, indicating a potential impact
of SKH on the lipid profile [5]. Similarly, another large study
showed e.g. that an increase in TSH levels of 1.0 m IU / L was
associated with an average increase in total cholesterol levels in
women of 0.09 mmol, indicating gender differences in the
relationship between SCH and lipid profile. Also, the relationship
between TSH levels and lipid profile is more pronounced with
advancing age [63].
Cardiovascular system, heart failure and ischemic heart
disease
SKH is associated with functional cardiac disorders, such as left
ventricular diastolic dysfunction and decreased systolic function at
rest and physical exertion [64]. Vascular abnormalities in this
condition have also been shown, such as increased vascular
resistance, arterial stiffness, endothelial dysfunction, and
atherosclerosis [65]. Many studies point to SKH as an independent
risk factor for the development of heart failure, as well as for the
worsening of existing ones [64].
Some of the results of research on the impact on ischemic heart
disease did not show an association between AITB and ischemic heart
disease, but by re-analyzing a population-based Whickham study (66),
it was found that in patients with SKH a significantly higher
frequency of cardiac ischemic events and mortality due to ischemic
heart disease was found. A meta-analysis of several relevant
prospective studies has shown similar results [67].
Degree of TSH increase
It is not insignificant, as the results of the study show, how much
TSH is elevated in SKH. We said that there are two categories of SKH
according to the degree of TSH increase: slightly elevated TSH, from
4-10 m IU/L and significantly elevated. TSH> 10 m IU/L. Symptoms,
manifestations, and potential complications, including endothelial,
lipid, and cardiovascular disorders, are related to the degree of
TSH elevation but depend, as has been said, on gender and age [68].
The results of numerous completed, as well as ongoing studies will
be useful to determine both the TSH threshold and the age threshold
for considering therapeutic intervention, levothyroxine
substitution.
THERAPEUTIC APPROACH IN SUBCLINICAL HYPOTHYROIDISM
SKH, like KH, is treated with levothyroxine substitution. The
goal of the treatment, as with KH, should be to eliminate the
symptoms of hypothyroidism by achieving normalization of TSH [69].
However, since it is by definition an asymptomatic disorder in most
patients, a disorder only at the blood level, two questions should
be kept in mind when deciding on the treatment: what is the effect
of levothyroxine treatment on long-term clinical outcomes in
patients with SLE and what is the outcome of follow-up without
levothyroxine treatment, on long-term outcomes in patients with SCV
[70]. Existing guidelines for the treatment of SKH differ from each
other, as there is conflicting evidence on the benefits of long-term
levothyroxine substitution in this condition. Although there are
data from several comprehensive reviews of the clinical outcomes of
SKH treatment, no definitive conclusion has yet been reached on the
benefits of this approach. (1). Certainly, as it was emphasized in
the previous text, before starting the substitution, the TSH test
should be repeated within 3 months from the diagnosis of SKH. This
is important because in about 60% of patients TSH normalizes within
3 months, and in about 62% over 5 years [71,44]. On the other hand,
in patients with SCC and hypothyroid symptoms, other possible causes
for existing symptoms should be considered first.
According to most guides, levothyroxine substitution in SKH should
be started when TSH is> 10 mIU/L, regardless of the absence of
symptoms. Levothyroxine substitution should be considered in cases
where TSH is between 5-10 mIU/L in repeated measurements and there
are symptoms similar to hypothyroidism. However, if symptoms do not
resolve after 3-4 months of levothyroxine substitution and TSH
normalization, the treatment should be discontinued [70,1]. In other
cases, the decision to treat SCH, when the TSH is between 5-10 mIU/L
in repeated measurements, should be adjusted individually depending
on age, comorbidity, degree of TSH elevation, persistence and
progression of TSH elevation, TAT presence and goiter. The meaning
of substitution would be based on reducing the risk of adverse CV
events and possibly preventing progression to CH. It should be borne
in mind that levothyroxine substitution can lead to iatrogenic
subclinical / clinical thyrotoxicosis, especially in elderly
patients, which in itself may be a risk of worsening CV condition
and there is no evidence that substitution is useful in people 65
years of age and older [42]. Factors that support the application of
left thyroxine therapy are: clinical trial due to symptoms of
hypothyroidism, patient’s desire, bipolar disorder, depression,
infertility / ovulatory dysfunction, presence of TAT, progressive
increase in TSH, pregnancy, or pregnancy planning, children,
adolescents.
RECOMMENDATIONS [3]
There are two categories of SKH according to TSH level: Slightly
elevated TSH - 4-10 m IU / L found in 90% of people with SKH; and
TSH> 10 m IU / L
The finding of elevated TSH with normal FT4 in the first measurement
should be repeated in 2-3 months, by re-measuring TSH, T4 and TPOAb
TSH and FT4 should be measured in individuals with elevated TPOAb /
TgAb and / or ultrasound indicating AIT
Age-specific reference ranges should be used to diagnose SKH in the
elderly population.
In patients younger than 65 years and with TSH> 10 m IU/L, even in
the absence of symptoms of hypothyroidism, the introduction of L-thyroxine
substitution is recommended.
In patients younger than 65 years with symptoms of hypothyroidism
and TSH <10 m IU/L, a clinical trial by introducing L-thyroxine
substitution should be considered.
After hemithyroidectomy, persistent SKH should be treated with L-thyroxine
in order to normalize TSH.
Patients with diffuse or nodular goiter and persistent SKH should be
treated with L-thyroxine in order to normalize TSH.
In patients with type 1 DM, TSH levels should be monitored once a
year.
In patients with DM type 2 and unexplained deterioration of glycemic
control, TSH and FT4 should be performed.
There is limited evidence that L-thyroxine substitution in younger
people with SKH leads to improved mental function.
There is no evidence of beneficial effects of L-thyroxine therapy in
obese individuals with TSH <10 m IU/L and normal FT4 on weight loss.
L-thyroxine therapy in SKH can lower both total and LDL cholesterol,
but lipid normalization is rarely achieved.
The effect of L-thyroxine substitution on serum lipid concentrations
is most pronounced in patients with TSH levels> 10 mIU/L before
treatment.
The oldest elderly people, over 80 years of age, with a TSH level ≤
10 m IU / L, should be carefully monitored, avoiding the
introduction of L-thyroxine substitution.
If the hormones in the control test are normal, with a normal TAT
level and the absence of goiter - no further testing is needed.
If SCH persists and L-thyroxine therapy is not started, hormones
should be tested for 6 months for at least first 2 years, and then
once a year.
PREGNANCY AND SUBCLINICAL HYPOTHYROIDISM
SKH in pregnancy is defined as a condition in which serum TSH is
higher than the upper limit of the reference range specific to the
trimester of pregnancy, while serum T4 and T3 are in the reference
ranges [72,73,14,74]. It occurs in approximately 2-2.5% of pregnant
women, with the number being significantly higher in some countries
and as high as 13.7% in northern Spain [75].
Isolated hypothyroxinemia is defined as a serum FT4 concentration
below the 2.5 percentile of the reference range (0.80 ng/dL;
10.30pmol/L), with a normal TSH concentration [72,12].
The diagnosis of SCH in pregnancy is made only on the basis of
laboratory analyses, as the symptoms and signs are non-specific and
very similar to problems that may be associated with lifestyle
variations, or problems that result from many other conditions and
pregnancy itself [72,12,74]. The reference range of TFT in pregnant
women differs from the reference range of the general population,
and also differs by trimesters of pregnancy. Based on published
studies, mainly in Western countries, the following reference range
for TSH in pregnancy is proposed: first trimester 0.1 - 2.5 mU/L;
second trimester 0.2 - 3.0 mU/L, third trimester 0.3–3.5 mU/L
[76-78]. However, it is advisable to determine these values for each
country or region individually. It should be noted that during
pregnancy there is an increase in the concentration of T4, which is
highest during the first trimester of pregnancy, while this increase
is significantly less during the second and third trimesters.
Despite the increased binding of hormones to transport proteins,
which are also increased in pregnancy, many authors believe that the
reliability of the determination of free thyroxine (FT4) by standard
immunoassay for FT4 is satisfactory [72,12].
As the definition of SKH is based on elevated TSH levels in
combination with normal FT4 values, it would be crucial to determine
the trimester-specific TH reference range. Available data from the
literature indicate that in the first trimester of pregnancy the
lower limit of FT4 2.5th percentile of the reference range detected
by immunoassays is about (0.80 ng/dL; 10.30pmol/L) [72,12]. In order
to obtain a reference value specific for the first trimester of
pregnancy, some authors suggest that the normal values of total, for
transport protein bound T4 (TT4), which are 5–12 mg / dL, or 50–150
nmol/L for non-pregnant women, be multiplied by 1.5 and the values
thus obtained used as reference values specific to the first
trimester [72,12].
Antibodies to thyroid peroxidase (TPOAb) are present in about 50% of
pregnant women with SCC, and up to 80% in pregnant women with
clinical hypothyroidism. In pregnant women with SCI, the
determination of TPOAb is recommended in order to determine the AITB.
Antibodies to thyroglobulin (TgAb) should not be neglected either.
Elevated TgAbs were found in 5% of women with SKH and normal TPOAb.
Women with elevated TgAb, and normal TPOAb, had significantly higher
serum TSH levels compared with women without AITB. Thus, TgAb should
be determined in pregnant women with negative TPOAb. After the first
trimester, TAT may be negative due to immunosuppression during
pregnancy, and in the presence of elevated TSH values and negative
antibodies, thyroid ultrasound should be performed [72,12].
Side effects of SKH during pregnancy
Manifested clinical hypothyroidism during pregnancy is clearly
associated with adverse events such as preeclampsia, eclampsia,
gestational hypertension, cretinism, fetal death, and miscarriage.
However, there is less evidence of complications during pregnancy
and SCI. Studies dealing with this problem show conflicting results.
Most studies indicate an increased risk of gestational diabetes (GD),
with a positive correlation between TSH levels and the risk of GD.
Several studies have confirmed the association of SKH with
miscarriages, very early embryo loss, gestational hypertension and
preeclampsia. The risk of preterm birth is also present in pregnant
women with SCI. Other complications that are mentioned as possible,
but also quite rare, are: placental abruption, increased perinatal
mortality, low Apgar score and low birth weight. However, the
association between SKH in pregnancy and offspring developmental
disorders has not been fully demonstrated [72,12].
Effects of SKH treatment during pregnancy
Treatment of SKH with levothyroxine is thought to outweigh the
potential benefits. SKH that occurs before conception, or during
gestation, should be treated with levothyroxine. In contrast, there
are no studies that show the benefit of treating isolated
hypothyroxinemia during pregnancy in terms of maternal obstetric
complications. However, levothyroxine therapy may be considered in
isolated hypothyroxinemia detected in the first trimester of
pregnancy, due to its association with more favorable
neuropsychological development in children. Levothyroxine therapy is
not recommended in isolated cases of hypothyroxinemia detected in
the second and third trimesters.
Levothyroxine therapy should be initiated in patients with TSH> 10
mU/l in the first trimester, regardless of the presence of TPOAb.
Also, therapy should be initiated in pregnant women with TSH> 4 mU/L
and TPOAb positive. Therapy should be considered in pregnant women
with TSH of 2.5-4mU/L with positive TPOAb and in pregnant women with
TSH of 2.5-10mU/L with negative TPOAb. In patients preparing for
pregnancy with assisted reproductive techniques, the TSH should be
<2.5 mU/L. In these patients, TSH should be determined two weeks
before and two weeks after insemination and in vitro fertilization (VTO)
[79].
If a decision is made to introduce substitution in pregnant women
with CKD, the suggested doses of levothyroxine are: 1.20 µg / kg /
day for TSH ≤ 4.2 mU / L; 1.42 µg / kg / day
for TSH> 4.2–10 m IU / L and 2.33 µg / kg / day for TSH> 10 mU / L.
TSH values should be checked every 4-6 weeks during the first
trimester and once during the second and third trimesters.
In patients with morning sickness, late levothyroxine administration
may be a legitimate option. The goal of levothyroxine treatment
during pregnancy is to normalize maternal serum TSH values within
trimester-specific reference values.
Most cases of SKH in pregnancy are transient and recover after
pregnancy. However, pregnant women with positive TPOAb and TSH> 5 mU/L
are more likely to have persistently elevated TSH, i.e. that
hypothyroidism will persist after pregnancy. After delivery, the
dose of levothyroxine should be reduced to the pre-conception dose.
In women diagnosed with SKH during pregnancy, whose TSH is <5 mU/L
and who have negative TPOAb, as well as in women whose replacement
dose was less than 50 µg of levothyroxine, discontinuation of
postpartum substitution may be attempted. Thyroid status checked 6
weeks postpartum, then at 6 and 12 months. In other women diagnosed
with SCC after pregnancy, thyroid status should be checked 6 months
and one year after delivery and the need for substitution should be
determined. Levothyroxine therapy is not recommended for euthyroid
women with positive antibodies [72,12]. Evidence for screening for
SKH in pregnancy is ambiguous. Although there are still no well-
controlled studies to justify general screening, a large number of
authors recommend screening. Also, a large number of authors
advocate screening only for pregnant women who are at special risk,
ie. women with a history of thyroid disease, women with a family
history of thyroid disease, women with goiter, women with DM type 1,
women with other autoimmune diseases, women with infertility of
unknown cause, women with a history of head and neck radiotherapy,
women with a history of abortion and premature birth [72,12,74,80].
SUBCLINICAL HYPOTHYROIDISM IN CHILDREN
The subject of our consideration is primarily SKH in adult
population, but we will make a few remarks about this condition in
children. When it comes to possible prenatal impact, the results of
numerous studies on the relationship between the mother's SKH and
impaired neurophysiological development of the child are not
consistent, as is very clear in KH [12], and further research is
needed to determine the exact impact. In newborns and early
childhood, especially in the first 3 years of life, THs play an
irreplaceable role in the process of maturation and brain
development, and the impact on linear growth persists until the
closure of the pineal gland in adolescence [81]. After birth, large
changes in thyroid function occur in the newborn, and the level of
TSH> 5 mU / L, can be considered elevated after 1 month of age.
Therefore, it is necessary, as in the elderly population, to use
age-specific reference values to interpret diagnostic biochemical
findings [82]. In the general pediatric and adolescent population
with SCH, hormones are normalized in over 70% of them, or persist
unchanged in most of the rest, for the next 5 years after the
diagnosis [12]. SKH is 10 times more common in children with Down
syndrome than in the general population [83]. In obese children, a
TSH level of 5-7 m IU/L is likely a consequence rather than a cause
of obesity [84]. In areas with sufficient iodine intake, SKH in
young children is most often idiopathic (so-called persistent "Hyperthyrotropinemia"
and "Non-autoimmune" idiopathic SKH), or caused by various perinatal
and genetic causes. In older children and adolescents, the most
common cause is AITB [12]. To date, there is insufficient evidence
to recommend levothyroxine substitution in most children with SKH
and TSH <10 mU/L [85].
AMIODARON-INDUCED SUBCLINICAL HYPOTHYROIDISM
Chronic therapy with amiodarone (AMD), an iodine-rich
antiarrhythmic, is associated with the appearance of predictable
changes in TFT, as well as the appearance of thyroid dysfunction,
which is responsible for both iodine load and cytotoxicity of the
antiarrhythmic [86]. According to research by the authors of this
paper, amiodarone-induced subclinical hypothyroidism (AISKH) is
found in the area with sufficient iodine intake in 10% of cardiac
patients treated with this antiarrhythmic, more often in women,
patients with enlarged thyroid gland and patients with elevated
TPOAb [87]. In most patients with AISK, the condition does not
progress to KH, and in a large number there is a spontaneous
normalization of thyroid status, even with continued amiodarone
therapy [88]. A case of amiodarone-induced thyrotoxicosis (AIT)
after AISC in a patient during continued amiodarone therapy has also
been described [89]. Also, during recovery from AIT, SKH may
develop, transient but also permanent [87,89]. It is recommended
that thyroid status be determined before initiating amiodarone
therapy and monitored regularly, usually every 6 months, during
therapy with this antiarrhythmic. In patients at increased risk for
thyroid dysfunction, i.e. women, patients with goiter and elevated
TAT, the use of another antiarrhythmic should be considered, or
thyroid status should be monitored more frequently. We believe that
it is not necessary to discontinue amiodarone therapy in AISCH, but
to continue regular monitoring of thyroid status [90,91].
MICRONUTRIENTS AND SUBCLINICAL HYPOTHYROIDISM
Life habits including sleep, smoking, diet, and physical activity
are significant factors influencing normal thyroid function in SKH
[92]. Iodine, selenium and iron are necessary for the synthesis of
thyroid hormones. Hem-bound iron is part of thyroid peroxidase (TPO),
which enables the incorporation of iodine atoms into tyrosine
molecules in the process of synthesis of thyroid hormones [93].
Myo-inositol, as a secondary messenger of phospholipase C, also
stimulates the organization of iodine and its incorporation into
thyroid hormones through the inositol phosphate / Ca 2+ /
diacylglycerol signaling pathway [94]. Selenium (daily requirements
are 55 µg, and in pregnancy and lactation 60-70 µg) as an integral
part of the enzyme deiodinase, enables the synthesis of
triiodothyronine, or inactivation of thyroxine by conversion to
reverse T3. In addition, selenoproteins, glutathione peroxidase, and
thioredoxin reductase affect iodine organization through their
effects on the concentration of reactive oxygen species,
particularly H 2 O 2., [93] .
Adequate iodine intake (about 150 μg per day), as well as adequate
TSH synthesis, are the basic prerequisites for the synthesis of
thyroid hormones. Iodine deficiency in the diet leads to reduced
synthesis of thyroid hormones, but its excessive intake has the same
effect, due to the Wolff-Chaikoff effect [94]. Due to the effect on
iodine organization, iron deficiency (daily requirements are about 9
mg for men and about 15 mg for menstruating women) affects thyroid
status as well as myo-inositol deficiency, which, unlike iron,
selenium and iodine, can still synthesize in the body from glucose,
so deficits are rare [94,95] . In the case of a combined deficiency
of iodine and selenium, in order to normalize the function of the
thyroid gland, it is necessary to first compensate for the
deficiency of iodine, and only after that the deficiency of selenium
[94,96].
CONCLUSION
SKH is a common condition and most do not require treatment, but
only follow-up. There is a consensus that levothyroxine substitution
should be indicated in adult patients with SCC whose TSH is ≥ 10 m
IU/L. In all other cases, the assessment is individual.
Recommendations regarding SCH screening vary widely among
professional associations and expert groups. Overall, screening is
not recommended in the general population and should be limited to
people at high risk for the condition, such as patients with
autoimmune diseases, positive personal or family history of thyroid
disease, and those with symptoms similar to hypothyroidism. Even in
asymptomatic pregnant women, opinions about the need for universal
screening are divided. Most professional associations suggest
targeted screening of only certain groups of patients.
LITERATURE:
- Bauer SB, Azcoaga-Lorenzo A, Agrawal U, McCowan C.
Management strategies forpatients with subclinical
hypothyroidism: a protocol for an umbrella review. Syst Rev
2021;10:290. https://doi.org/10.1186/s13643-021-01842-y BMC
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH,
et al. Subclinicalthyroid disease: scientific review and
guidelines for diagnosis and management. Jama. 2004;291:228–38.
- Simon H.S. Pearce HSS, Brabant G, Duntas HL, Monzani F,
Peeters PR, Salman Razvi S, Wemeau JL. 2013 ETA Guideline:
Management of Subclinical Hypothyroidism. Eur Thyroid J
2013;2:215–228. DOI: 10.1159/000356507
- Gharib H, Tuttle MR, H. Baskin J, Fish HL, Singer AP,
McDermott TM. Consensusstatement: Subclinical Thyroid
Dysfunction: A Joint Statement on Management from the American
Association of Clinical Endocrinologists,the American Thyroid
Association, and The Endocrine Society. J Clin Endocrinol Metab
2005; 90(1):581–585.
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC: The Colorado
thyroid disease prevalence study. Arch Intern Med 2000; 160:
526–534.
- Vanderpump MP, Tunbridge WM, French JM, et al: The incidence
of thyroid disorders in the community: a twenty-year follow-up
of the Whickham Survey. Clin Endocrinol 1995; 43: 55–68.
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter
EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid
antibodies in the United States population (1988 to 1994):
National Health and Nutrition Examination Survey (NHANES III) J
Clin Endocrinol Metab. 2002;87:489–499.
- Zimmermann BM, Boelaert K. Iodine deficiency and thyroid
disorders. Lancet Diabetes Endocrinol 2015;3:286–95.
http://dx.doi.org/10.1016/ S2213-8587(14)70225-6
- Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of Subclinical
Hypothyroidism in Patients with Metabolic Syndrome. Endocrine
Journal 2007;54(1):71–76.
- Han C, He X, Xia X, Li Y, Shi X, Shan Z, Teng W.Subclinical
Hypothyroidism and Type 2 Diabetes: A Systematic Review and
Meta-Analysis.PLoS One. 2015;10(8):e0135233.
- Chonchol M, LippiG, Salvagno G, Zoppini G, Muggeo M, Targher
GConclusions: These findings suggest that subclinical primary
hypothyroidism is a relatively common condition (∼18%) among
persons with CKD not requiring chronic dialysis, and it is
independently associated with progressively lower estimated GFR
in a large cohort of unselected outpatient adults.. Prevalence
of Subclinical Hypothyroidism in Patients with Chronic Kidney
Disease. Clin J Am Soc Nephrol. 2008; 3(5):1296–1300. doi:
10.2215/CJN.00800208
- Lazarus J, Brown SR, Daumerie C, Hubalewska-Dydejczyk A,
Negro R, Vaidya B. Guidelines for the Management of Subclinical
Hypothyroidism in Pregnancy and in Children. Eur Thyroid J
2014;3:76–94. DOI: 10.1159/000362597
- Surks IM, Boucai L. Age- and Race-Based Serum Thyrotropin
Reference Limits. J Clin Endocrinol Metab 2010;95(2):496–502.
https://doi.org/10.1210/jc.2009-1845
- Hennessey VJ, Espaillat R. Subclinical hypothyroidism: a
historical view and shifting prevalence. Int J Clin Pract. 2015;
69(7):771–782. doi: 10.1111/ijcp.12619
- Dittmar M, Kahaly GJ. Polyglandular autoimmune syn-dromes:
immunogenetics and long-term follow-up. J Clin Endocrinol Metab.
2003;88:2983-2992.
- Broadley SA, Deans J, Sawcer SJ, Clayton D, Compston DA.
Autoimmune disease in first-degree relatives of patients with
multiple sclerosis. A UK survey. Brain. 2000;123:1102-1111.
- Heward J, Gough SC. Genetic susceptibility to the
development of autoimmune disease. Clin Sci (Lond). 1997;93:
479-491.
- Menconi F, Monti MC, Greenberg DA, et al. Molecular amino
acid signatures in the MHC class II peptide-binding pocket
predispose to autoimmune thyroiditis in humans and in mice. Proc
Natl Acad Sci USA. 2008;105:14034-14039.
- Ban Y, Greenberg DA, Davies TF, Jacobson E, Concepcion E,
Tomer Y. Linkage analysis of thyroid antibody production:
evidence for shared susceptibility to clinical autoimmune
thyroid disease. J Clin Endocrinol Metab. 2008;93:3589-3596.
- Andersson M, de Benoist B, Delange F, Zupan J. Prevention
and control of iodine deficiency in pregnant and lactating women
and in children less than 2-years-old: conclusions and
recommendations of the Technical Consultation. Public Health
Nutr. 2007;10:1606-1611.
- Emerson CH, Dysno WL, Utiger RD. Serum thyrotropin and
thyroxine concentrations in patients receiving lithium
carbonate. J Clin Endocrinol Metab. 1973;36:338-346.
- Preziati D, La Rosa L, Covini G, et al. Autoimmunity and
thyroid function in patients with chronic active hepatitis
treated with recombinant interferon alpha-2a. Eur J Endocrinol.
1995;132:587-593.
- Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects
of amiodarone on the thyroid. Endocr Rev. 2001;22:240-254.
- Kappers MH, van Esch JH, Smedts FM, de Krijger RR, et al.
Sunitinib-induced hypothyroidism is due to induction of type 3
deiodinase activity and thyroidal capillary regression. J Clin
Endocrinol Metab. 2011;96:3087-3094.
- Santen RJ, Misbin RI. Aminoglutethimide: review of
pharmacology and clinical use. Pharmacotherapy1981;1(2):95-120.
- Matveyeva SL, Shevchenko OS, Pogorelova OO. The function of
the thyroid gland in patients with multi-drug resistant
tuberculosis. Antimicrobial Resistance and Infection Control
2017;6:82-84. DOI 10.1186/s13756-017-0238-4
- Moreno DM, Miguélez González M, González Fernández L,
Percovich Hualpa HC. A review of systemic infiltrative diseases
and associated endocrine diseases
Endocrinología,DiabetesyNutrición (English ed.) 2021;68:312-320.
- Ozen Oz Gul, Soner Cander, Canan Ersoy. . An uncommon
infiltrative disease of thyroid: Riedel's thyroiditis. Endocrine
Abstracts 2014; 35:P282. DOI: 10.1530/endoabs.35.P282
- Payami H, Joe S, Thomson G. 1989 Autoimmune thy-roid disease
in type I diabetic families. Genet Epidemiol. 1989;6:137-141.
- Nerup J. Addison’s disease—clinical studies. A report of 108
cases. Acta Endocrinol (Copenh). 1974;76:127-141.
- Torfs CP, King MC, Huey B, Malmgren J, Grumet FC. Genetic
interrelationship between insulin-dependent diabetes mellitus,
the autoimmune thyroid diseases, and rheumatoid arthritis. Am J
Hum Genet. 1986;38:170-187.
- Murdoch JC, Ratcliffe WA, McLarty DG, Rodger JC, Ratcliffe
JG. Thyroid function in adults with Down’s syndrome. J Clin
Endocrinol Metab. 1977;44:453-458.
- Radetti G, Mazzanti L, Paganini C, et al. Frequency,
clinical and laboratory features of thyroiditis in girls with
Turner’s syndrome. The Italian Study Group for Turner’s
Syndrome. Acta Paediatr. 1995;84:909-912.
- Mouat F, Evans HM, Cutfield WS, Hofman PL, Jefferies C.
Massive hepatic hemangioendothelioma and consumptive
hypothyroidism. J Pediatr Endocrinol Metab. 2008;21:701-703.
- Robin P. Peeter. Subclinical Hypothyroidism. N Engl J Med
2017;376:2556-2565. DOI: 10.1056/NEJMcp1611144
- Huber G, Staub JJ, Meier C, et al: Prospective study of the
spontaneous course of subclinicalhypothyroidism: prognostic
value of thyrotropin, thyroid reserve, and thyroid antibodies. J
Clin Endocrinol Metab 2002;87:3221–3226.
- Diez JJ, Iglesias P: Spontaneous subclinical hypothyroidism
in patients older than 55 years: an analysis of natural course
and risk factors for the development of overt thyroid failure. J
Clin Endocrinol Metab 2004; 89:4890–4897.
- Meyerovitch J, Rotman-Pikielny P, Sherf M, et al: Serum
thyrotropin measurements in the community: five-year follow-up
in a large network of primary care physicians. Arch Intern Med
2007;167:1533–1538.
- Walsh JP, Bremner AP, Feddema P, et al. Thyrotropin and
thyroid antibodies as predictors of hypothyroidism: a 13-year,
longitudinal study of a community-based cohort using current
immunoassay techniques. J. Clin. Endocrinol. Metab.
2010;95:1095–1104.
- Kalaria T, Sanders A, Fenn J, et al. The diagnosis and
management of subclinical hypothyroidism is assay-dependent–
Implications for clinical practice. Clin. Endocrinol. (Oxf).
2021;94:1012–1016.
- Surks MI & Hollowell JGAge-specific distribution of serum
thyrotropin and antithyroid antibodies in the US population:
implications for the prevalence of subclinical hypothyroidism.
J. Clin. Endocrinol. Metab. 2007;92:4575–4582.
- Biondi B, Cappola AR & Cooper DS. Subclinical
Hypothyroidism: A Review. JAMA 2019;322:153–160.
- Hattori N, Ishihara T, Yamagami K, et al. Macro TSH in
patients with subclinical hypothyroidism. Clin. Endocrinol.
(Oxf). 2015;83:923–930.
- Koulouri O, Moran C, Halsall D, et al. Pitfalls in the
measurement and interpretation of thyroid function tests. Best
Pract. Res. Clin. Endocrinol. Metab. 2013;27:745.
- Santini F, Marzullo P, Rotondi M, et al. Mechanisms in
endocrinology: the crosstalk between thyroid gland and adipose
tissue: signal integration in health and disease. Eur. J.
Endocrinol. 2014;171:R137–R152.
- Kim WG, Park S, Jeon MJ, et al. Clinical Features of Early
and Late Postoperative Hypothyroidism After Lobectomy. J. Clin.
Endocrinol. Metab. 2017;102:1317–1324.
- Ardabilygazir A, Afshariyamchlou S, Mir D, et al. Effect of
High-dose Biotin on Thyroid Function Tests: Case Report and
Literature Review. Cureus 2018;10.
- Katzman BM, Lueke AJ, Donato LJ, et al. Prevalence of biotin
supplement usage in outpatients and plasma biotin concentrations
in patients presenting to the emergency department. Clin.
Biochem. 2018;60:11–16.
- Garber JR, Cobin RH, Gharib H, et al: Clinical practice
guidelines for hypothyroidism in adults: cosponsored by the
American Association of Clinical Endocrinologists and the
American Thyroid Association. Thyroid 2012;22:1200–1235.
- Pedersen OM, Aardal NP, Larssen TB, et al: The value of
ultrasonography in predicting autoimmune thyroid disease.
Thyroid 2000;10:251–259.
- Andersen S, Pedersen KM, Bruun NH, Laurberg P: Narrow
individual variations in serum T 4 and T 3 in normal subjects: a
clue to the understanding of subclinical thyroid disease. J Clin
Endocrinol Metab 2002;87:1068–1072.
- Bremner AP, Feddema P, Leedman PJ, et al: Age-related
changes in thyroid function: a longitudinal study of a
community-based cohort. J Clin Endocrinol Metab 2012;97:
1554–1562.
- Persani L, Borgato S, Romoli R, et al: Changes in the degree
of sialylation of carbohydrate chains modify the biological
properties of circulating thyrotropin isoforms in various
physiological and pathological states. J Clin Endocrinol Metab
1998;83:2486–2492.
- Asvold BO, Bjoto T, Vatten LJ: Association of serum TSH with
high body mass differs between smokers and never-smokers. J Clin
Endocrinol Metab 2009;94:5023–5027.
- Villar HC, Saconato H, Valente O, Atallah AN: Thyroid
hormone replacement for subclinical hypothyroidism. Cochrane
Database Syst Rev 2007;3:CD003419.
- Samuels MH, Schuff KG, Carlson NE, et al: Health status,
mood, and cognition in experimentally induced subclinical
hypothyroidism. J Clin Endocrinol Metab 2007;25:2545– 2551.
- Parle J, Roberts L, Wilson S, et al: A randomized controlled
trial of the effect of thyroxine replacement on cognitive
function in community- living elderly subjects with subclinical
hypothyroidism: the Birmingham Elderly Thyroid Study. J Clin
Endocrinol Metab 2010;95:3623–3632.
- Kitahara CM, Platz EA, Ladenson PW, et al: Body fatness and
markers of thyroid function among US men and women. PLoS One
2012;7:e34979.
- Fox CS, Pencina MJ, D’Agostino RB, et al: Relations of
thyroid function to body weight: cross-sectional and
longitudinal observations in a community-based sample. Arch
Intern Med 2008;168:587–592.
- Wolters B, Lass N, Reinehr T: TSH and freetriiodothyronine
concentrations are associated with weight loss in a lifestyle
interventionand weight regain afterwards in obese children. Eur
J Endocrinol 2013;168:323–329.
- Maratou E, Hadjidakis DJ, Kollias A, et al: Studies of
insulin resistance in patients with clinical and subclinical
hypothyroidism. Eur J Endocrinol 2009;160:785–790.
- Triolo TM, Armstrong TK, McFann K, et al: Additional
autoimmune disease found in 33% of patients at type 1 diabetes
onset. Diabetes Care 2011;34:1211–1213.
- Tognini S, Polini A, Pasqualetti G, et al: Age and gender
substantially influence the relationship between thyroid status
and the lipoprotein profile: results from a large
cross-sectional study. Thyroid 2012;22:1096–1103.
- Biondi B: Mechanisms in endocrinology: heart failure and
thyroid dysfunction. Eur J Endocrinol 2012;167:609–618.
- Shakoor SK, Aldibbiat A, Ingoe LE, et al: Endothelial
progenitor cells in subclinical hypothyroidism: the effect of
thyroid hormone replacement therapy. J Clin Endocrinol Metab
2010;95:319–322.
- Vanderpump MP, Tunbridge WM, French JM, et al: The
development of ischemic heart disease in relation to autoimmune
thyroid disease in a 20-year follow-up study of an English
community. Thyroid 1996;6:155– 160.
- Ochs N, Auer R, Bauer DC, et al: Meta-anal ysis: subclinical
thyroid dysfunction and the risk for coronary heart disease and
mortal ity. Ann Intern Med 2008;148:832–845.
- Rodondi N, den Elzen WP, Bauer DC, et al. Thyroid Studies
Collaboration: Subclinical hypothyroidism and the risk of
coronary heart disease and mortality. JAMA 2010;304:1365–1374.
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.;
Cappola, A.R.; et al. Guidelines for the treatment of
hypothyroidism: Prepared by the american thyroid association
task force on thyroid hormone replacement. Thyroid
2014;24;1670–1751.
- Calissendor J, Falhammar H. To Treat or Not to Treat
Subclinical Hypothyroidism, What Is the Evidence? Medicina
2020;56:40. doi:10.3390/medicina56010040
- Stott D.J., Rodondi N., Kearney P.M., Ford I.,Westendorp
R.G.J. et al. Thyroid hormone therapy for older adults with
subclinical hypothyroidism. N. Engl. J. Med. 2017;376:2534–2544.
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H,Dosiou
C, et al. 2017 Guidelines of the American Thyroid Associationfor
the Diagnosis and Management of Thyroid Disease During Pregnancy
and the Postpartum.Thyroid. 2017;27(3):315-389.
- Brenda S. Bauer, Amaya Azcoaga-Lorenzo, Utkarsh Agrawal and
Colin McCowan. Management strategies for patients with
subclinical hypothyroidism: a protocol for an umbrella review.
Syst Rev 2021;10:290.
- Galina Khachikovna Safarian, Alexander Mkrtichevich Gzgzyan,
Kharryasovna Dzhemlikhanova Lyailya and Dariko Alexandrovna
Niauri. Does subclinical hypothyroidism and/or thyroid
autoimmunity influence the IVF/ICSI outcome? Review of the
literature. Gynecological Endocrinology. 2019;35(Sup1):56-59.
- Aguayo A, Grau G, Vela A, Aniel-Quiroga A, Espada M, Martul
P, Castano L, Rica IJ: Urinary iodine and thyroid function in a
population of healthy pregnant women in the North of Spain.
Trace Elem Med Biol 2013;27:302–306.
- Haddow JE, Palomaki GE, McClain MR: Thyroid-stimulating
hormone in singleton and twin pregnancy: importance of
gestational age-specific reference ranges. Obstet Gynecol
2006;107:205–206.
- Soldin OP, Soldin D, Sastoque M: Gestationspecific thyroxine
and thyroid stimulating hormone levels in the United States and
worldwide. Ther Drug Monit 2007;29:553–559.
- Haddow JE, McClain MR, Lambert-Messerlian G, Palomaki GE,
Canick JA, et al. First and Second Trimester Evaluation of Risk
for Fetal Aneuploidy Research Consortium: Variability in
thyroid-stimulating hormone suppression by human chorionic
gonadotropin during early pregnancy. J Clin Endocrinol Metab
2008;93:3341-3347.
- Kris Poppea, Peter Bisschopb Laura Fugazzolac, Gesthimani
Minziorid, David Unuanee Andrea Weghofer. 2021 European Thyroid
Association Guideline on Thyroid Disorders prior to and during
Assisted Reproduction. Eur Thyroid J. 2020;9:281–295.
- Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison
S, et al. Detection of thyroid dysfunction in early pregnancy:
Universal screening or targeted high-risk case finding? J Clin
Endocrinol Metab. 2007;92(1):203–7.
- Brown RS: The thyroid; in Brook CGD, Clayton PE, Brown RS
(eds): Brook’s Clinical Pediatric Endocrinology, ed 6.
Chichester, Wiley-Blackwell, 2009; pp 250–282.
- Chaler EA, Fiorenzano R, Chilelli C, Llinares V, Areny G,
Herzovich Vet al.: Age-specific thyroid hormone and thyrotropin
reference intervals for a pediatric and adolescent population.
Clin Chem Lab Med 2012; 50: 885–890.
- King K, O’Gorman C, Gallagher S: Thyroid dysfunction in
children with Down syndrome: a literature review. Ir J Med Sci
2014;107:118–119.
- Ittermann T, Thamm M, Wallaschofski H, Rettig R, Volzke H:
Serum thyroid-stimulating hormone levels are associated with
blood pressure in children and adolescents. J Clin Endocrinol
Metab 2012; 97: 828–834.
- Aijaz NJ, Flaherty EM, Preston T, Bracken SS, Lane AH,
Wilson TA: Neurocognitive function in children with compensated
hypothyroidism: lack of short term effects on or off thyroxin.
BMC Endocr Disord 2006;6:2.
- Aleksić Ž, Aleksić A, Mitov V, Jolić A, Vešović D. Vrednosti
in vitro pokazatelja funkcijskog tiroidnog statusa kod
pacijenata na terapiji Amiodaronom. Medicinski glasnik Zlatibor.
2012;17(44 Suppl):90.
- Aleksić Ž, Aleksić A.. Incidenca amiodaronom indukovanih
tiroidnih disfunkcija i prediktivni faktori za njihov nastanak.
Timočki medicinski glasnik 2011;36(Suppl 1):28.
- Aleksić Ž, Aleksić A. Amiodaronom indukovan supklinički
hipotiroidizam. Timočki medicinski glasnik 2015;40(Suppl 1):31.
- Aleksić Ž, Aleksić A, Mitov V, Jolić A, Vešović D.
Amiodaronom indukovana tirotoksikoza kod prethodno subklinički
hipotiroidnog pacijenta na terapiji amiodaronom – prikaz
slučaja. Timočki medicinski glasnik 2012;37(Suppl 1):92.
- Aleksić Ž. Subklinički hipotiroidizam – dijagnostičke i
terapijske dileme. Timočki medicinski glasnik 2018;43(Suppl
1):38.
- Aleksić ŽP, Aleksić AZ, Mitov VM, Jolić AD, Vešović DM.
Amiodarone induced subclinical thyroid dysfunction – what to
expect during follow up? Is there reason for amiodarone
withdrawal? Eur Thyroid J 2012;1(suppl 1):188.
- Wu K, Zhou Y, Ke S, et al.Lifestyle is associated with
thyroid function in subclinical hypothyroidism: a
cross-sectional study. BMC Endocr. Disord. 2021;21:1–11.
- Zimmermann MB & Köhrle J. The impact of iron and selenium
deficiencies on iodine and thyroid metabolism: biochemistry and
relevance to public health. Thyroid 2002;12:867–878.
- Benvenga S, Nordio M, Laganà AS, et al.The Role of Inositol
in Thyroid Physiology and in Subclinical Hypothyroidism
Management. Front. Endocrinol. (Lausanne) 2021;12:458.
- Soliman AT, De Sanctis V, Yassin M, et al.Chronic anemia and
thyroid function. Acta Bio Medica Atenei Parm. 2017;88:119.
- Ventura M, Melo M & Carrilho F. Selenium and Thyroid
Disease: From Pathophysiology to Treatment. Int. J. Endocrinol.
2017:1297658. https://doi.org/10.1155/2017/1297658
|
|
|
|

