| |
|
|
Introduction
Coronary calcifications occur when calcium builds up in the
plaque of the coronary arteries. They are more common in the
elderly, in patients with diabetes, renal insufficiency, as well as
with previous cardiovascular revascularization [1,2]. Calcified
coronary artery lesions continue to represent a challenge in
interventional cardiology. Fourteen studies with drug-eluting stents
showed that the frequency of moderately to severely calcified
lesions is about 30% of the total number of lesions. Calcified
coronary arteries are a sign of advanced atherosclerosis, associated
with multivessel disease and the presence of complex lesions,
including long lesions, chronic total occlusions, and bifurcations
[3]. Accumulated mineral content in calcified plaque increases the
frequency of complications during the procedure by obstructing
passage and leading to asymmetric or incomplete expansion of
balloons and stents, also leading to malposition of stents,
increasing postprocedural complications such as restenosis and stent
thrombosis [4,5].
This paper presents a patient with a calcified lesion of the ostium
of the anterior descending artery (left anterior descending, LAD)
and percutaneous coronary intervention (PCI) with the help of
rotational atherectomy (RA).
Case report
An 83-year-old female patient was admitted to our institution due
to acute myocardial infarction with inferior ST segment elevation .
The complaints started an hour before admission. This was the first
manifestation of coronary disease. The patient was previously
treated for arterial hypertension and diabetes. Immediately after
admission, an emergency selective coronary angiography was
performed, which registered an occluded right coronary artery (RCA)
with a significant calcified lesion of the LAD, as well as the
ostium of the ramus intermedius (RI). In the same act, primary PCI
RCA was performed with the implantation of two drug-eluting stents
with a flap (2.75x12mm, 2.75x18mm). Echocardiographically,
hypokinesia of the basal half of the inferior wall and the inferior
septum and the apical third of the anterior septum was registered,
with preserved global systolic function. The patient was treated
with dual antiplatelet therapy, low-molecular-weight heparin, beta
blocker, angiotensin-converting enzyme inhibitor, dihydropyridine
calcium channel blocker, statin, and antidiabetic therapy was
optimized. The medical documentation was presented to the
cardiosurgical council, which indicated surgical revascularization
of the myocardium with double aortocoronary bypass (LAD and RI),
which the patient refused, and PCI LAD and RI was proposed to her.
In the second act, during the same hospitalization, PCI was
attempted. Predilatation of the RI ostium was performed with a
2.5x15mm semi-compliant balloon. An attempt to predilate the LAD
ostium with a non-compliant balloon 3.5x15mm, as well as with
semi-compliant balloons 2.0x15mm and 1.5x10mm was not successful,
because the balloons did not pass the calcified lesion. Given that
no dissection was registered in the left coronary system, that the
patient had anginal complaints all the time, was hemodynamically and
rhythmologically stable, and electrocardiographically without signs
of ischemia, further intervention was abandoned and an attempt at RA
of the ostial LAD with eventual PCI of the LAD was indicated.
One month after the acute event, the patient was readmitted to our
institution for a planned intervention. The intervention was
performed through the right femoral approach. The main stem is
cannulated with a guide catheter EBU (Eng. Extra Back-Up) 3.5 7F. A
working wire was passed through the lesion and placed in the distal
segment of the LAD. Via the microcatheter, the Corsair Pro working
wire was replaced with an Extra Support Rota wire. A rotablation of
the calcified lesion of the ostium was performed with a 1.5mm LAD
burr at 150,000 rpm (eng. rotation per minute) with three
repetitions of a maximum duration of up to 15s. Rota wire was
replaced by working wire. A second working wire is positioned in the
distal segment of the RI for protection. The ostial LAD lesion was
then predilated with a non-compliant 3.0x20mm balloon. Two flap
drug-eluting stents were implanted from the main stem to the LAD
(3.5x22mm, 3.0x30mm) with proximal optimization of the stent in the
main stem with a non-compliant balloon 5.0x15mm. An optimal
angiographic result with normal coronary flow was obtained. The
patient was discharged on the third day of hospitalization without
complications.
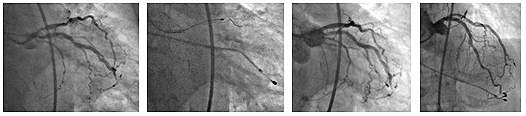
Pictures 1. Angiographic findings before the
procedure; 2. RA calcified lesions of the LAD ostium; 3. and 4.
Angiographic findings after the procedure
Discussion
Several non-invasive and invasive methods can be used to diagnose
calcified lesions of the coronary arteries: computed tomography
coronary angiography (CTCA), selective coronary angiography,
intravascular ultrasound (IVUS) and optical coherence tomography
tomography, OCT). Selective coronary angiography often
underestimates calcified lesions, and with this method it is not
possible to assess the depth of calcium in the plaque [6]. On
fluoroscopy, coronary calcification is radio-opaque, it is observed
before contrast injection, and it is mostly a circumferential lesion
[7]. IVUS and OCT are two invasive methods that provide better data
on the depth and distribution of calcium in the plaque. The
characteristics of the lesion that we can obtain using OCT, which
may suggest that treatment with RA will be needed, are: maximum
circumference of the calcification >180°, maximum thickness >0.5mm,
length >5mm [8]. An indication for RA can be the impossibility of
passage of the lesion with balloons or insufficient expansion of the
balloon when preparing the lesion for PCI.
Today, there are several strategies used to modify calcified lesions
before the PCI procedure and can be divided into non-atherectomy and
atherectomy strategies. Strategies without atherectomy include
modification balloons (non-compliant, so-called scoring, so-called
cutting balloons) as well as intravascular lithotripsy. These
methods treat the lesion by fracture, cutting, or targeted
dissection. Atherectomy strategies are aimed at physical plaque
removal and include RA, coronary orbital atherectomy, laser coronary
atherectomy [9].
RA is an endovascular procedure during which plaque ablation occurs
by advancing a rotating abrasive burr. This method has been around
for three decades, but is extremely rarely used in clinical
practice. According to the available data, the use of RA in Europe
and the USA is in 1-3% of the total number of PCI procedures [10].
Although randomized trials with both metal [11] and drug-eluting
stents [12,13] did not show a reduced incidence of long-term
ischemic events with the routine use of RA, the use of RA in
severely calcified lesions is associated with a higher by expanding
the diameter of the blood vessel, with a larger cross-section of the
lumen and with fewer final residual stenoses after stent
implantation [14]. In 2018, the results of the PREPARE-CALC study
were published, which showed the non-inferiority of RA compared to
modification balloons in terms of in-stent lumen loss nine months
after PCI with the implantation of modern drug-eluting stents, as
well as the superiority of RA in terms of procedural success [15].
The main indication for the use of RA is the modification of
severely calcified coronary lesions with the aim of preparing the
lesion for further angioplasty and stent implantation. It is more
often used during re-intervention, but retrospective comparisons
have shown that, if RA is used as the primary method, the duration
of the procedure is reduced (average reduction 19 min), fluoroscopy
time (average reduction 18 min), as well as the volume of iodine
contrast medium used (average reduction reduction 70ml) [16].
Absolute contraindications for this method include CTO that prevents
wire passage, vein graft, acute thrombosis, shock and hypotension.
The presence of coronary artery dissection is not an absolute
contraindication. Care should be taken with severe left ventricular
dysfunction, severe coronary disease, disease of the unprotected
main stem, lesion length over 25mm, and lesion angle >45° [17].
As for ostial and bifurcation lesions, they are often more demanding
to work with, with possible plaque transfer, acute side branch
occlusion, and suboptimal stent apposition or expansion. In such
cases, interventions with the modification of the calcified plaque
with the use of RA have been shown to be more successful, whether
only the main branch or both the main and side branches are treated
[18,19,20,21].
When choosing a guide catheter, the 6F system is adequate for a burr
size of 1.75 mm and smaller. A 7F guide catheter is required for a
larger burr. The transradial approach is associated with a similar
success rate as the transfemoral approach [22,23]. Passage of the
lesion with a Rota wire is possible but challenging. An initial
passage with a working wire that can then be replaced via a
microcatheter with a Rota wire is an easier way to pass the lesion
itself. If it is not possible to pass the lesion with a
microcatheter, then you should try primarily to pass the lesion with
a Rota wire, and then, in case of successful passage, do the RA with
the smallest burr of 1.25 mm. Rota wires are available in two
versions, Extra Support and Floppy. Extra Support Rota wire is used
in ostial and distal lesions for better support [24]. The size of
the burr for RA is determined by the size of the blood vessel in
which the lesion is located. The results of the STRATAS and CARAT
studies indicate that a smaller burr (burr size ratio: coronary
artery <0.7) enables angiographic and procedural success equivalent
to a larger burr, with fewer complications [25,26]. It is
recommended to use a burr in which the ratio of the size to the size
of the artery to be treated is 0.4-0.6 [24]. In addition to choosing
the optimal size, a successful procedure also requires an adequate
rotation speed of the burr (140000 to 150000 rpm), with short
ablations (<20s) and pauses between ablations, as well as avoiding a
drop in rotation speed for more than 5000 rpm. The RA is considered
complete when the last burr maneuver passes without resistance.
After successful RA, implantation of a drug-eluting stent is
recommended. A follow-up of 1176 patients treated for RA from 2002
to 2013 showed that patients treated with drug-eluting stents had a
>50% lower risk of a major adverse cardiovascular event [27].
In our institution, about 20 RAs are performed per year, with a
success rate of 95%. All procedures are indicated after previously
unsuccessful attempts at PCI. In this case, RA was performed after
an unsuccessful attempt to pass the smallest balloon through the
calcified lesion of the ostial LAD. The procedure was performed
through a transfemoral approach using a 7F guide catheter, Extra
Support Rota wire, a 1.5mm burr with a rotation speed of 150000 rpm.
After successful RA, drug-eluting stents were implanted.
CONCLUSION
Carefully performed rotational atherectomy can be successfully
used in the treatment of demanding calcified lesions of the ostial
segments of the coronary arteries with a high degree of
effectiveness and safety. The use of other complementary methods
together with rotary atherectomy increases the success of the
procedure.
LITERATURE:
- Tomey MI, Kini AS, Sharma SK. Current status of rotational
atherectomy. JACC Cardiovasc Interv 2014;7:345–53.
- Sharma SK, Tomey MI, Teirstein PS, et al. North American
Expert Review of Rotational Atherectomy. Circ Cardiovasc Interv
2019;12:e007448.
- Carlotta SD, Giulia N, Francesca R, Alessio M, Brunilda H,
Carlo DM. Contemporary Approach to Heavily Calcified Coronary
Lesions. Interventional Cardiology Review 2019;14(3):154–63.
- Takebayashi H, Kobayashi Y, Mintz GS, Carlier SG, Fujii K,
Yasuda T, Moussa I, Mehran R, Dangas GD, Collins MB, Kreps E,
Lansky AJ, Stone GW, Leon MB, Moses JW. Intravascular ultrasound
assessment of lesions with target vessel failure after sirolimus-eluting
stent implantation. Am J Cardiol. 2005; 95:498–502. doi:
10.1016/j.amjcard.2004.10.020
- Kobayashi Y, Okura H, Kume T, Yamada R, Kobayashi Y,
Fukuhara K, Koyama T, Nezuo S, Neishi Y, Hayashida A, Kawamoto
T, Yoshida K. Impact of target lesion coronary calcification on
stent expansion.Circ J.2014 ; 78:2209–2214.
- Wang X, Matsumura M, Mintz GS, et al. In vivo calcium
detection by comparing optical coherence tomography,
intravascular ultrasound, and angiography. Am J Coll Cardiol
Imaging 2017;10:869–79.
- Moussa I, Ellis SG, Jones M, Kereiakes DJ, McMartin D,
Rutherford B, Mehran R, Collins M, Leon MB, Popma JJ, Russell
ME, Stone GW. Impact of coronary culprit lesion calcium in
patients undergoing paclitaxel-eluting stent implantation (a
TAXUS-IV sub study). Am J Cardiol.2005; 96:1242–1247.
- Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M,
Usui E, Yonetsu T, Haag ES, Shlofmitz RA, Kakuta T, Maehara A. A
new optical coherence tomography-based calcium scoring system to
predict stent underexpansion. .EuroIntervention.2018;
13:e2182–e2189.
- Tanush G, Michael W, Mark G, Antonio C, Azeem L. Rotational
Atherectomy: A Contemporary Appraisal. Interventional Cardiology
Review 2019;14(3):182–9.
- Barbato E, Carrie D, Dardas P, et al. European expert
consensus on rotational atherectomy. EuroIntervention
2015;11:30–6.
- Dill T, Dietz U, Hamm CW, Küchler R, Rupprecht HJ, Haude M,
Cyran J, Ozbek C, Kuck KH, Berger J, Erbel R. A randomized
comparison of balloon angioplasty versus rotational atherectomy
in complex coronary lesions (COBRA study) . Eur Heart J. 2000;
21:1759–1766.
- Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist
V, Meinertz T, Schofer J, King L, Neumann FJ, Khattab AA.
High-speed rotational atherectomy before paclitaxel-eluting
stent implantation in complex calcified coronary lesions: the
randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent
Treatment for Complex Native Coronary Artery Disease) trial.JACC
Cardiovasc Interv.2013; 6:10–19.
- de Waha S, Allali A, Büttner HJ, Toelg R, Geist V, Neumann
FJ, Khattab AA, Richardt G, Abdel-Wahab M. Rotational
atherectomy before paclitaxel-eluting stent implantation in
complex calcified coronary lesions: two-year clinical outcome of
the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;
87:691–700.
- Hoffmann R, Mintz GS, Popma JJ, Sattler LF, Kent KM, Pichard
AD, Leon MB. Treatment of calcified coronary lesions with Palmaz-Schatz
stents. An intravascular ultrasound study. Eur Heart J. 1998;
19:1224–1231.
- Mohamed Abdel-W, Ralph T, Robert AB, Walker G, Mohamed El-M,
et al. High-Speed Rotational Atherectomy Versus Modified
Balloons Prior to Drug-Eluting Stent Implantation in Severely
Calcified Coronary Lesions. The Randomized PREPARE-CALC Trial.
Circulation: Cardiovascular Interventions. 2018;11:e007415.
https://doi.org/10.1161/CIRCINTERVENTIONS.118.007415
- Kawamoto H, Latib A, Ruparelia N, Boccuzzi GG, Pennacchi M,
Sardella G, Garbo R, Meliga E, D'Ascenzo F, Moretti C, Rossi ML,
Presbitero P, Ielasi A, Magri C, Nakamura S, Colombo A. Planned
versus provisional rotational atherectomy for severe calcified
coronary lesions: insights from the rotate multi-center
registry. Catheter Cardiovasc Interv. 2016; 88:881–889.
- Boston Scientific Corporation. Rotational Atherectomy System
Reference Guide. in 2014
- Karvouni E, Di Mario C, Nishida T, Tzifos V, Reimers B,
Albiero R, Corvaja N, Colombo A. Directional atherectomy prior
to stenting in bifurcation lesions: a matched comparison study
with stenting alone. Catheter Cardiovasc Interv.2001; 53:12–20.
- Tsuchikane E, Aizawa T, Tamai H, Igarashi Y, Kawajiri K,
Ozawa N, Nakamura S, Oku K, Kijima M, Suzuki T; PERFECT
Investigators. Pre-drug-eluting stent debulking of bifurcated
coronary lesions. J Am Coll Cardiol.2007; 50:1941–1945.
- Nageh T, Kulkarni NM, Thomas MR. High-speed rotational
atherectomy in the treatment of bifurcation-type coronary
lesions. Cardiology. 2001; 95:198–205.
- Ito H, Piel S, Das P, Chhokar V, Khadim G, Nierzwicki R,
Williams A, Dieter RS, Leya F. Long-term outcomes of plaque
debulking with rotational atherectomy in side-branch ostial
lesions to treat bifurcation coronary disease.J Invasive
Cardiol.2009; 21:598–601.
- Kotowycz MA, Khan SQ, Freixa X, Ivanov J, Seidelin PH,
Overgaard CB, Džavík V. Rotational atherectomy through the
radial artery is associated with similar procedural success when
compared with the transfemoral route. Coron Artery Dis. 2015;
26:254–258.
- Watt J, Oldroyd KG. Radial versus femoral approach for
high-speed rotational atherectomy. Catheter Cardiovasc Interv.
2009; 74:550–554.
- Samin S, Matthew T, Paul T, Annapoorna K, Arthur R, Arthur
L, Philippe G, Jeffrey C, Cindy G, Stevan H, Craig T, Ian M,
Aparna B, Jeffrey M. North American Expert Review of Rotational
Atherectomy. Circulation: Cardiovascular Interventions Vol. 12,
No. 5, 2019;12:e007448.
- Whitlow PL, Bass TA, Kipperman RM, Sharaf BL, Ho KK, Cutlip
DE, Zhang Y, Kuntz RE, Williams DO, Lasorda DM, Moses JW, Cowley
MJ, Eccleston DS, Horrigan MC, Bersin RM, Ramee SR, Feldman T
Results of the study to determine rotablator and transluminal
angioplasty strategy (STRATAS).Am J Cardiol.2001; 87:699–705.
- Safian RD, Feldman T, Muller DW, Mason D, Schreiber T, Haik
B, Mooney M, O'Neill WW. Coronary angioplasty and rotablator
atherectomy trial (CARAT): immediate and late results of a
prospective multicenter randomized trial. Catheter Cardiovasc
Interv. 2001; 53:213–220.
- Kawamoto H, Latib A, Ruparelia N, Ielasi A, D'Ascenzo F,
Pennacchi M, Sardella G, Garbo R, Meliga E, Moretti C, Rossi ML,
Presbitero P, Magri CJ, Nakamura S, Colombo A, Boccuzzi GG.
In-hospital and midterm clinical outcomes of rotational
atherectomy followed by stent implantation: the ROTATE
multicentre registry.EuroIntervention.2016; 12:1448–1456.
|
|
|
|

