| |
|
|
Introduction
Medicinal plants can be used in a raw state, but there are a lot
of products made by their processing and they are named
phytopreparations. All of them can be divided in a few categories:
cosmetics, dietary supplements (food), medical devices and
medicines. According to the Serbian Drugs Law, the herbal medicine
means a product which as an active ingredient contains one or more
plants (in total or in parts) in a dry or fresh form or their
untreated exudates; or which contains herbal preparations (got by
pharmaceutical-technological treatment of herbal material) or a
combination of these two categories. Except herbal medicines, in the
group of medicines that contain the herbal material as active
ingredient we can find traditional herbal medicines and homeopathic
medicines. Magistral and galenic medicines are specific groups of
medicines which can contain the herbal material as active ingredient
too [1]. It is important to note that medicines which contain
isolated phytomolecules do not belong to the group of herbal
medicines.
Due to their complex content, the formulation of herbal medicines is
very demanding from pharmaceutical-technological and posological
standpoint. They often have several constituents responsible for the
pharmacological effect, which, sometimes, are not fully recognized
[2.3]. However, only herbal remedies prepared in accordance with the
principles of rational phytotherapy have acceptable quality, which
then guarantees their safety and efficiency [4]. This implies the
formulation of the dosage form with precisely defined active
principles and their standardization [5]. Phytopreparations that are
registered as herbal medicines should meet these requirements to the
fullest extent.
Common problem with preparations that as active principle contain
plants or their products is a lack of evidences of efficiency. The
possible reason for this is opportunity registration as
non-medicines, and this only requires a safety certificate in most
countries. However, there are studies that unambiguously confirm the
efficacy for precisely defined indications of these group medicines.
This creates the opportunity for bigger use of these drugs, and
prescribing effective therapy with a reasonable level of side
effects, of which patients would have the most of benefit [6,7].
In general, clinical efficacy of these medicines is less than
evidenced efficacy in in-vitro conditions often due to the
undesirable biopharmaceutical profile of active constituents. Poor
lipid solubility and improper molecular size influence the final
bioavailability, and consequently lead to reduced pharmacological
efficacy. Great therapeutic potential of plants can be utilized by
applying a novel drug delivery system during formulation of herbal
medicines. The novel formulations have a significant advantage
compared to the conventional formulations. Beside to efficacy, these
systems improve other two standards important for each medicine:
safety and quality. They are good enhancers of biopharmaceuticals
properties (solubility and permeability) and efficacy. They also
facilitate delivering active constituents to the pharmacological
target. Additionally, the novel drug delivery systems decrease
toxicity, thus increasing the safety of herbal medicines. They
protect from physical and chemical degradation and enhance stability
[8] Some of existing novel drug delivery systems which can be used
for formulation of herbal medicines are:
Liposomes
Liposomes are created from one, several or multiple concentric
bilayer membranes and hydrophilic core. They belong to the group of
colloid, bearing in mind that sizes of their particles are between
0.05 and 5 μm in diameter. The main advantage of liposomes is the
ability to deliver almost all groups of molecules: hydrophilic,
lipophilic, macromolecules and small molecules. This can be
especially important for herbal extracts containing a complex
mixture of compounds [8-10].
Phytosomes
Phytosomes involve the formation of a chemical bond between the
bilayer membrane component and the plant extract component. This
leads to a significant increase in absorption. The difference in
relation to liposomes is that the active principle here is an
integral part of the membrane [11,12].
Ethosomes/Transferosomes
Ethosomes are built from phospholipids and high concentrations of
ethanol (20-45 %). Beside to ethanol, transfersomes are composed of
surfactants that facilitate delivery of medicines. These systems are
excellent carriers in transdermal drug delivery systems (TDDS)
because successfully broke barrier function of skin [8-10].
Microemulsions
Emulsions are biphasic systems in which one phase is dispersed in
other phase. One phase must be water (or aqueous phase), while other
one must be oily liquid (non-aqueous). Generally, we can divide
emulsions by size of dispersed phase at: ordinary emulsions (0.1-100
μm), micro-emulsions or nano-emulsions (10-100 nm), and
sub-micro-emulsion or lipid emulsions (100-600 nm). Micro-emulsions
are thermodynamically stable and they are suitable carriers for
lipophilic medicines [8-9].
Nanocapsules and Nanospheres
These novel drug delivery systems belong to a group of polymeric
nanoparticles (PNPs) and they are made by biocompatible and
biodegradable polymers. The nanospheres have a matrix form in which
the active principle is located, and the nanocapsules are made of
two separate parts: membranes and core in which the active principle
is. This type of novel system is excellent in overcoming the lack of
conventional dosage forms. They significantly increase the efficacy
and reduce a required dose [12-14].
The aim of the study was to determine the specificity of herbal
medicines present on the market of Serbia in the period from 2006 to
2016.
METHOD
Data which were used in the study were taken from the official
website of Agency for Medicines and Medical Devices of Serbia.
Annual reports on the Turnover and Consumption of Medicines Intended
for Human Use (Chapter 7b: Natural and financial overview of the
achieved turnover of herbal medicines in the Republic of Serbia,
according to the Anatomical and Chemical Classification of Herbal
Medicines (HATC) and the International Unprotected Drug Name (INN))
were observed for the period from 2006 to 2016 (these reports have
been available online so far) [15-25]. Data about consumption were
delivered to the agency by drug manufacturers or their
representatives. Marketing Authorization Holder gives data about
prices of medicines whose regime for issuing is without physician's
prescription.
The conversion of consumption (expressed in Serbian dinars) in euros
was made in accordance with the middle yearly exchange rate
available on the website of the National Bank of Serbia [26].
Data analysis and graph drawing were done using the Microsoft Excel
programme.
RESULTS AND DISCUSSION
Turnover of herbal and traditional medicines in 2016 compared to
2006 was increased by 15.67 times or by 1466.33 %. Medicines from
HATC groups R, N, G (registered from 2014) and A were had the
highest turnover. Graph 1 shows turnover of herbal and traditional
medicines in the observed period.
Graph 1. Turnover expressed in euros, divided by
HATC groups
Grafikon 1. Promet izražen u eurima, podeljen po HATC grupama
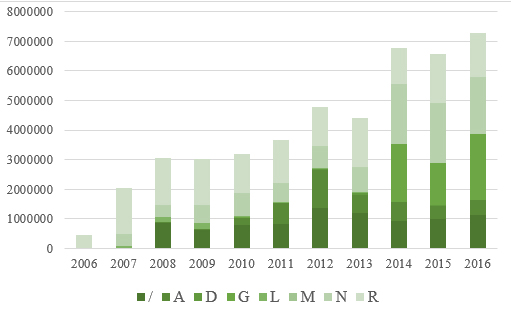
A significant percentage of medicines are not classified
according to the HATC system, which points to the need for
additional efficacy studies that would facilitate the classification
of this group of drugs.
Another key point is that herbal and traditional herbal medicines
turnover were increased consumption share in total medicines
turnover (Graph 2). The share of herbal and traditional drugs in the
consumption of all medicines increased from 0.09% (2006) to 0.82%
(2016), i.e. the increase of share is by 9.11 times or by 811.11%
Graph 2. Share of herbal and traditional medicines
in total consumption of medicines
Grafik 2. Udeo biljnih i tradicionalnih lekova u ukupnoj potrošnji
lekova
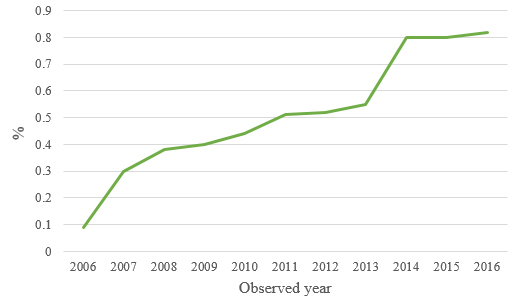
An increased rate of use of plants for the treatment of various
diseases exists in the world too. Global botanical and plant-derived
drugs’ market have increasing trend and it was: $23.2 billion in
2013, $24.4 billion in 2014 and $25.6 billion in 2015. It is
expected that it will be $35.4 billion in 2020 and will have a
compound annual growth rate of 6.6% from 2015 to 2020 [27]. It is
known that about 80% of people in developing countries use plants
for treatment, but there is a significant rate of use of these drugs
in developed countries. Research says that more than 50% of the
population of developed countries (Europe, North America) use herbal
medicine at least once in their lifetime [28]. In Germany and
Canada, this percentage is between 70% and 90% [29]. Such a trend
does not surprise because, apart from the low rate of side effects
(in short-term use), good efficacy, low cost and good compliance,
herbal medicines could potentially be used for the formulation of
preparations for the treatment of very serious diseases such as
asthma [30], HIV infection [31] or cancer [32].
It is suggested that the reasons for the increasing trend in the use
of herbal remedies may be: consumer preferences for natural and
alternative therapies, dissatisfaction with conventional therapy,
affinity for self-medication, low cost, belief that these drugs are
more effective and less dangerous, but also the modernization of
pharmaceutical forms [33]. In addition, because these drugs belong
to the group of OTC products for which advertising is permitted, an
increasing number and quality of advertisements lead to increased
use [34].
At the same time, the increase in the use of herbal medicines in
Serbia is characterized by a poor knowledge about this group of
medicinal products [35].
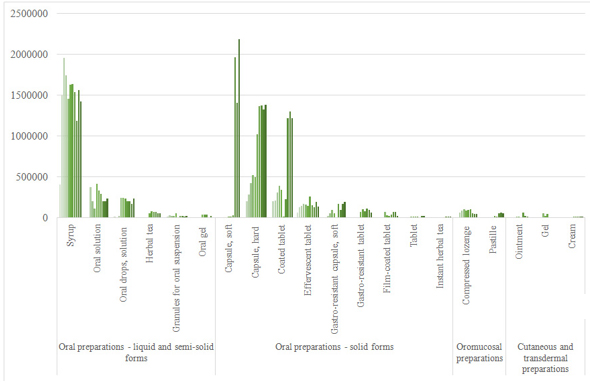
Analyzed data of used pharmaceutical forms in the observed period
are shown in graph 3. As can be seen, there is downward trend in
turnover of liquid pharmaceutical dosage forms, and upward trend
turnover of solid forms. Generally, liquid forms are less stable
than solid and additionally solid forms are more acceptable for use
in most people. Soft capsules had the highest consumption
(especially in the last observed year), and topical preparations had
very low turnover. When we talk about oromucosal preparations,
compressed lozenges had higher turnover, but with downward trend,
the opposite of pastilles.
This significant increase in the use of herbal medicines was not
followed by an increase in the presence of contemporary
pharmaceutical dosage forms, since in the observed period there were
no registered herbal (or traditional) medicines formulated by novel
drug delivery system. Definitely, we can say that modern forms of
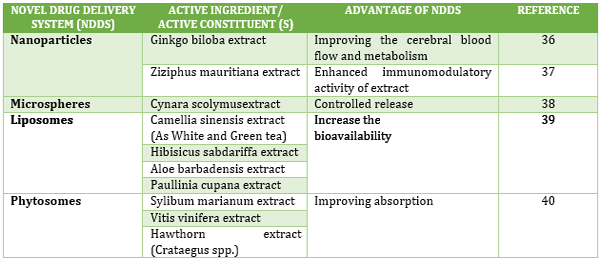
herbal medicines are not the cause of increasing their use. In other
countries herbal products formulated with the help of novel drug
delivery systems are not just part of the scientific researches, but
they also exist in the market. Some of them are shown in Table 1.
Table 1. Existing modern forms of herbal
preparations in other countries
Tabela 1. Postojeći moderni oblici biljnih preparata u drugim
zemljama
Graph 3. Turnover of different dosage forms of
herbal (and traditional) medicines
Grafik 3. Promet različitih oblika doziranja biljnih (i
tradicionalnih) lekova
CONCLUSION
Improving the quality of health care implies the application of
guidelines for rational pharmacotherapy and phytotherapy. This
implies giving the right medication, in the right dose for the right
patient, right time and right route. Following the trends of
developed countries (primarily Germany), the use of herbal drugs
could be even greater. If herbal medicines on the Serbian market
should be formulated according to the latest scientific research,
the effectiveness of these drugs would be even greater.
Acknowledgement
This study was supported by the Grants of the Ministry of Education,
Science and Technological Development of the Republic of Serbia,
projects No 172058 and No 41012.
Conflict of interest
There is no conflict of interest.
REFERENCES:
- Zakon o lekovima i medicinskim sredstvima. Službeni glasnik
RS, 2010, br. 30 [in Serbian]. Available at:
www.lat.rfzo.rs/down load/zakon_lekovi-lat.pdf.
- Schulz V, Hansel R, Tyler VE. Rational phytotherapy. A
physician’s guide to herbal medicine. 4thedn. Springer-Verlag.
Berlin. 2001.
- Kunle, FolashadeO,Egharevba, Omoregie H, Ahamadu, Ochogu P.
Standardization of herbal medicines-A Review. Int J
BiodiverConser. 2012;4(3):101-12.
- Colalto C. What phytotherapy needs: Evidence-based
guidelines for better clinical practice.Phytother Res. 2018
Mar;32(3):413-25.
- Djordjevic SM. From Medicinal Plant Raw Material to Herbal
Remedies. In: Aromatic and Medicinal Plants-Back to Nature 2017.
InTech.
- Ernst E. The efficacy of herbal medicine–an overview.
FundamClinPharmacol. 2005 Aug;19(4):405-9.
- Maiti B, Nagori BP, Singh R. Recent trends in herbal drugs:
a review. IntJ of Drug Res and Technol. 2017 Apr 25;1(1).
- Saraf S. Applications of novel drug delivery system for
herbalformulations. Fitoterapia. 2010;81(7):680-9.
- Chaturvedi M, Kumar M, Sinhal A, Saifi A. Recent development
in novel drug delivery systems of herbal drugs. Intl J Green
Pharm. 2011;5(2).
- Ambwani S, Tandon R, Ambwani TK, Malik YS. Current knowledge
on nanodelivery systems and their beneficial applications in
enhancing the efficacy of herbal drugs. J ExpBiolAgric Sci. 2018
Feb 1;6(1):87-107.
- Singh B, Awasthi R, Ahmad A, Saifi A. Phytosome: most
significant tool for drug deliveryto enchance
thetherapeuticbenefits ofphytoconstituents. J Drug Deliv Therap.
2018 Jan 15;8(1):98-102.
- Patil RY, Patil SA, Chivate ND, Patil YN. Herbal Drug
Nanoparticles: Advancements in Herbal Treatment. Res J
PharmTechnol. 2018;11(1):421-6.
- Khalil NM. Phytosomes: A Novel Approach for Delivery of
Herbal Constituents. 2018.
- Ramos MA, Da Silva PB, Spósito L, De Toledo LG, Bonifácio
BV, Rodero CF, Dos Santos KC, Chorilli M, Bauab TM.
Nanotechnology-based drug delivery systems for control of
microbial biofilms: a review. Int J Nanomedicine. 2018;13:1179.
- *Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2006. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2007.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2007. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2008.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2008. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2009.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2009. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2010.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2010. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2011.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2011. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2012.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2012. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2013.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2013. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2014.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2014. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2015.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2015. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2016.
- Radonjić V. Promet i potrošnja gotovih lekova za humanu
upotrebu u 2016. godini. Beograd: Agencija za lekove i
medicinska sredstva Srbije. 2017.
- National Bank of Serbia [Internet]. Exchange Rate Lists For
a Specifi c Period. [cited 2018 Nov 11]. Available at:
http://www.nbs.rs/export/sites/default/internet/english/scripts/kl_period.html.
- BCC Research report. Botanical and Plant-derived Drugs:
Global Markets. Report Buyer. (Accessed Aug 2015). 2015.
Available at:
https://www.reportbuyer.com.
- **Gunjan M, Naing TW, Saini RS, Ahmad A, Naidu JR, Kumar I.
Marketing trends & future prospects of herbal medicine in the
treatment of various disease. World Journal of Pharmaceutical
Research. 2015;4(9):132-55.
- Khan MS, Ahmad I. Herbal Medicine: Current Trends and Future
Prospects. InNew Look to Phytomedicine 2019 Jan 1 (pp. 3-13).
Academic Press.
- Huntley A, Ernst E. Herbal medicines for asthma: a
systematic review. Thorax. 2000 Nov 1;55(11):925-9.
- Bekut M, Brkić S, Kladar N, Dragović G, Gavarić N, Božin B.
Potential of selected Lamiaceae plants in anti (retro) viral
therapy. Pharmacological research. 2017 Dec 16.
- Roy A, Attre T, Bharadvaja N. Anticancer agent from
medicinal plants: a review. 2017.
- Bandaranayake WM. Quality control, screening, toxicity, and
regulation of herbal drugs. Modern phytomedicine: turning
medicinal plants into drugs. 2006. pp. 25-57.
- Parle M, Bansal N. Herbal medicines: are they safe? 2006.
- Samojlik I, Mijatović V, Gavarić N, Krstin S, Božin B.
Consumers’ attitude towards the use and safety of herbal
medicines and herbal dietary supplements in Serbia.
International journal of clinical pharmacy. 2013 Oct
1;35(5):835-40.
- Shimada S. Composition comprising nanoparticle Ginkgo biloba
extract with the effect of brain function activation IPC8
Class-AA61K914FI, USPC Class-424489; 2008.
- Bhatia A, Shard P, and Chopra D, Mishra T: Chitosan
nanoparticles as carrier of immunorestoratory plant extract:
synthesis, characterization and immunorestoratory efficacy.
International journal of drug delivery 2011; 3:381-5.
- Gavini E, Alamanni MC, Cossu M, Giunchedi P. J Microencapsul
2005;22(5):487–99.
- Liposome Herbasec® [Internet]. Liposomal encapsulated,
standardized botanicals in powder form.[Cited 2018 Nov 16]
Available at:
https://www.in-cosmetics.com/__novadocuments/2319
- Integrative Therapeutics [Internet]. Super milk thistle ® X.
[cited 2018 Nov 16]. Available at:
https://www.integrativepro.com/Products/Gastrointestinal/Super-Milk-Thistle
|
|
|
|




