| |
|
|
INTRODUCTION
A key feature of the menopausal transition is the reduction in
estradiol levels [1]. Consequently, many of the components of
metabolic syndrome (MetS) (central obesity, dyslipidemia, impaired
fasting glucose and hypertension) are often seen in that period [2],
and numerous studies have shown that being affected by MetS
increases the risk, as well as morbidity of cardiovascular disease
(CVD) [3, 4].
The prevalence of the MetS is increasing rapidly throughout the
world. Studies about MetS have shown that females were more affected
than males [5]. Moreover, the prevalence of MetS increases in women
after menopause [6].
Although the MetS is multifactorial in origin, impaired glucose
tolerance, dyslipidemia, and hypertension are caused by the same
underlying mechanism-endothelial dysfunction primarily mediated by
oxidative stress. A growing body of evidence suggests that increased
oxidative stress to adipocytes is central in the pathogenesis of CVD
in MetS [7].
On the other hand, uric acid is regarded as independent risk factor
for CVD [8]. In addition, elevated uric acid impairs endothelial
function by inducing intracellular oxidative stress and inflammation
through activation of the local renin-angiotensin system, and the
pro-oxidant effect of uric acid per se, once absorbed into
endothelial cells [9].
In line with this, it is speculated that uric acid is one of the
determinants of the MetS [10]. The elevated serum uric acid level
observed in the MetS has been attributed to hyperinsulinemia, since
insulin reduces renal excretion of uric acid [11].
Data concerning the prevalence of MetS among postmenopausal women in
Montenegro are limited. Therefore, the aim of this study was to
evaluate the prevalence of this syndrome and to examine its
association with serum uric acid, an established cardiometabolic
risk factor.
Materials and methods
STUDY POPULATION
The study enrolled a total of 242 women (ages 43-68 years) who
developed menopause and who volunteered to participate in the study.
Participants were consecutively recruited in the study when seeking
gynaecologic healthcare in the Primary Health Care Centre in
Podgorica, in a period from October 2012 to May 2013. All the
participants completed a questionnaire including demographic
characteristics, somatic illnesses, medications use, and lifestyle
habits. Menopause is defined as having last menstrual cycle for more
than one year. Inclusion criteria were: women without signs and
symptoms of acute inflammatory disease, with no history or the
presence of malignancy, hypo- and hyperthyroidism, or CVD.
Participants who had gout, renal dysfunction, hepatic dysfunction,
cardiovascular disorders were excluded from the study, as well as
those who used anti-inflammatory medications or hormonal replacement
therapy. The participants were instructed not to perform any
vigorous physical activity the day before the blood samples were
taken. Medical history and clinical examinations were carried out on
the same day. All the participants provided written informed
consent. The study protocol was approved by Ethical Committee of
Primary Health Care Centre in Podgorica and the research was carried
out in compliance with the Declaration of Helsinki [12].
Anthropometric measurements
Basic anthropometric measurements: body height (cm), body weight
(kg) and waist circumference (WC) (cm) were obtained twice in the
morning on the same day and the mean values were used for the
analysis. Body mass index (BMI) was calculated as weight in
kilograms divided by height in meters squared (kg/m2). All
measurements were taken by the same trained evaluator. Blood
pressure was measured with a sphygmomanometer after the subject had
been seated for 15 minutes. The average of three measurements taken
on the right arm was recorded.
The participants were divided into two groups: group of
postmenopausal women without MetS (n=140), and group of
postmenopausal women with MetS (n=102). MetS was diagnosed according
to the modified US National Cholesterol Education Program Adult
Treatment Panel III (NCEP ATP III) guidelines [13]. The participants
met with at least three or more of the following conditions were
diagnosed with MetS:
- Waist circumference (WC) ≥ 88 cm;
- Hyperglicemia ≥5.6mmol/L or antidiabetic medication use
(instead of previous cut-off values of fasting plasma glucose
≥6.1mmol/L);
- High density lipoprotein cholesterol (HDL-c) ≤1.29mmol/L;
- Triglycerides ≥1.70mmol/L or lipid lowering medication use;
- Hypertension: systolic blood pressure (SBP) or diastolic
blood pressure (DBP) ≥130/85 mm
Hg and/or diagnosed hypertension treated with antihypertensive
therapy.
Biochemical analyses
Blood samples were taken between 7-9 hours a.m., after 12-14 hours
of an overnight fast. Samples were left to clot for 30 minutes and
then centrifuged at 3000 rpm for 10 minutes. Serum levels of
glucose, total cholesterol (TC), high density lipoprotein
cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c),
triglycerides (TG) and uric acid level were measured using
standardized enzymatic procedures using a spectrophotometer (Roche
Cobas 400, Mannheim, Germany).
Statistical analysis
Statistical analyses were performed using SPSS statistical package
(version 15.0 for Windows, SPSS, Chicago, IL, USA). Data are
presented as mean ± standard deviation or median (interquartile
range), or counts and percentages. Differences in uric acid level
between groups were evaluated with a Student's t test, and χ²-test
was used for categorical variables. Receiver Operating
Characteristic (ROC) curve analysis was used with the purpose of
testing discriminatory capability of uric acid with MetS status as
dependent variable. In all analyses, P value of < 0.05 was
considered as statistically significant.
RESULTS
Table 1 shows the general clinical and biochemical
characteristics of the study participants.
Data are presented as mean ± standard deviation or median
(interquartile range), or counts and percentages; BMI-Body mass
index; WC-Waist circumference; HDL-c-High density lipoprotein
cholesterol; LDL-c-Low density lipoprotein cholesterol;
TG-Triglycerides; SBP-Systolic blood pressure; DBP-Diastolic blood
pressure
The prevalence of MetS and its components is presented in Table 2.
MetS is diagnosed in 42.1% of women. As shown, the most prevalent
feature was abdominal obesity (65.3%), following by hypertension
(systolic blood pressure (SBP) and diastolic blood pressure (DBP)
accounting for 54.1% and 49.2% respectively), hyperglycemia (40.5%),
and dyslipidemia (high triglycerides and low HDL-c level accounting
for 30.2%, and 25.2% respectively).
Table 1. General characteristics of studied postmenopausal
women
Tabela 1. Opšte karakteristike ispitivanih žena u
postmenopauzi.
| Characteristics |
Postmenopausal women (n=242) |
| Age (years) |
56.7± 4.52 |
| BMI (kg/m²) |
27.2±4.00 |
| WC (cm) |
92.7±12.43 |
| Fasting glucose (mmol/L) |
5.40 (5.10-6.00) |
| Total cholesterol (mmol/L) |
6.24±1.12 |
| HDL-c (mmol/L) |
1.56 (1.29-1.83) |
| LDL-c (mmol/L) |
3.89 (3.25-4.58) |
| TG (mmol/L) |
1.37 (0.99-1.76) |
| Uric acid (µmol/L) |
276±62.0 |
| SBP (mm Hg) |
133±21.3 |
| DBP (mm Hg) |
83.7±12.3 |
| Current smokers % (n) |
16.9 (41) |
| Antihypertensive drugs % (n) |
36.0 (87) |
| Lipid lowering drugs % (n) |
13.2 (32) |
| Hypoglycemic drugs % (n) |
19.4 (47) |
Table 2. The prevalence of metabolic syndrome and its
components among postmenopausal women
Tabela 2. Prevalenca metaboličkog sindroma i njegovih
komponenti kod žena u postmenopauzi.
| The prevalence of MetS and its components among
postmenopausal women |
% |
| MetS |
42.1 |
| WC ≥ 88 cm |
65.3 |
| Fasting glucose ≥ 5.6 mmol/L |
40.5 |
| HDL-c ≤ 1.29 mmol/L |
25.2 |
| TG ≥ 1.70 mmol/L |
30.2 |
| SBP ≥ 130 mm Hg |
54.1 |
| DBP ≥ 85 mm Hg |
49.2 |
MetS-Metabolic syndrome; WC-Waist circumference; HDL-c-High
density lipoprotein cholesterol; TG-Triglycerides; SBP-Systolic
blood pressure; DBP-Diastolic blood pressure
In the current study we also aimed to test the association of MetS
status with uric acid level. We found significantly higher number of
patients with MetS compared to subjects without diagnosed MetS
across uric acid tertiles (χ2=27.02, P<0.001)), (Table 3). This
confirmed, at least partially, the association of uric acid with
MetS status in postmenopausal women in our study.
In addition, we found significantly higher uric acid levels were in
postmenopausal women with MetS than in those without MetS (304±61.1
vs. 256±54.7 µmol/L, P<0.001), (Graph 1).
Table 3. Metabolic syndrome distribution in uric acid
tertiles values subgroups
Tabela 3. Distribucija metaboličkog sindroma prema tercilnim
vrednostima mokraćne kiseline.
| |
Uric acid tertiles values |
P* |
| MetS status |
I tertile (n=99)
≤251 µmol/L |
II tertile (n=106)
252-350 µmol/L |
III tertile (n=37)
≥350 µmol/L |
| MetS +, n (%) |
24 (23.5%) |
52 (51.0%) |
26 (25.5%) |
χ2=27.02
P<0.001 |
| MetS -, n (%) |
75 (53.6%) |
54 (38.6%) |
11 (7.9%) |
MetS+ women with Metabolic syndrome; MetS- women without
Metabolic syndrome
Graph 1. Serum uric acid levels in
postmenopausal women according to metabolic syndrome status
Dijagram 1. Serumske vrednosti mokraćne kiseline u odnosu na
prisustvo metaboličkog sindroma.
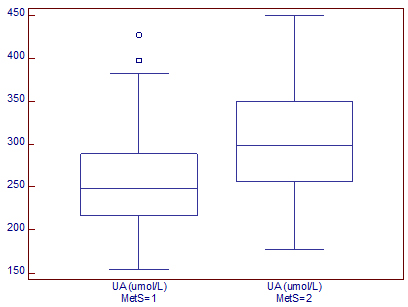
UA-uric acid; MetS=1-women without metabolic syndrome; MetS=2-women
with metabolic syndrome
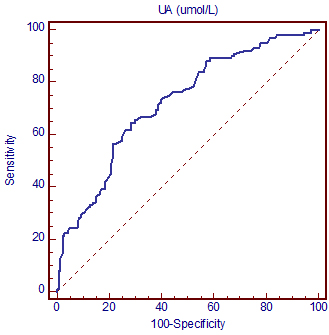
Thereafter, we conducted a receiver operating characteristic
(ROC) analysis to test the discriminatory capability of uric acid
regarding MetS status. Figure 1 shows ROC curve graph and Table 4
shows the most important ROC parameters: area under the curve (AUC)
with 95% confidence interval (CI) of selected parameter.
ROC curve showed good discriminatory capability (AUC=0.722,
according to Hosmer and Lemeshow’s rules) toward the MetS status
[14].
Table 4. Area under the curve, 95% Confidence Interval and
Standard error for the uric acid discriminatory capability regarding
the metabolic syndrome status
Tabela 4. Površina ispod krive, 95% Interval poverenja i
standardna greška diskriminacione moći mokraćne kiseline u odnosu na
metabolički sindrom.
| Parameter |
AUC 95% |
CI |
SE |
Sensitivity (%) |
Specificity (%) |
P |
| Uric acid |
0.722 |
0.661-0.777 |
0.034 |
64.7 |
71.4 |
<0.001 |
AUC - area under ROC curve; CI- Confidence interval; SE-standard
error
Figure 1. ROC curve of the uric acid
discriminatory capability regarding the metabolic syndrome status
Slika 1. ROC kriva diskriminacione moći mokraćne kiseline u
odnosu na metabolički sindrom.
DISCUSSION
The incidence of MetS among postmenopausal women was found to be
drastically increased in the world. In the current study the
prevalence of MetS was 42.1%, with abdominal obesity as the most
prevalent feature (65.3%) which is similar with the results of the
other studies [15]. The high prevalence of MetS among postmenopausal
women in the world varies from 32.6% in Austria, to 54.6% in South
Korea [16]. In Chinese postmenopausal women the prevalence of MetS
was 33.7% [17]. According to a study of Pandey et al. [18] the
prevalence of MetS among Indian postmenopausal women was 55%.
Moreover, MetS was highly prevalent among Brasilian postmenopausal
women [19], and the most prevalent risk factor was abdominal
obesity, affecting 62.5% of women. MetS also seems to be a major
health problem among postmenopausal women in many developing
countries, like Bangladesh, accounting for 39.3% of postmenopausal
women having MetS. Even more, the prevalence of MetS was 1.78 times
higher in postmenopausal women than in premenopausal ones [19]. Neto
et al. [6] found that women between 40 and 45 years had a prevalence
of MetS of 14.1%, while for women between 56 and 64 years the
prevalence was even 66.7%.
The obtained results of the current study are unexpected. Taking
into account that Montenegro is the part of Mediterranean basin, and
that Mediterranean-type dietary pattern would be expected as the
preferable one due to easy access of consumers to the Mediterranean
products, these results are discouraging. The possible explanation
for this may be the sedentary lifestyle and unhealthy dietary
pattern, with the increasing prevalence of obesity.
It is important to note that this prevalence would be expected to be
even higher if we take into consideration the International Diabetes
Federation criteria (IDF), with lower WC cut-off values ≤80 cm [20]
than reported in the current study.
In addition to the assessment of traditional risk factors, as
components of MetS, we have been explored serum uric acid level and
showed its good discriminatory capability toward MetS status. In
line with previous studies [21-23] we reported higher serum uric
acid level in women with MetS, comparing to those without MetS.
Moreover, in a prospective study conducted by Zurlo et al. [24] high
serum uric acid levels significantly and independently predicted
MetS in older women, but not in men, over a 4.4-year follow-up.
The underlying mechanism of the association between serum uric acid
levels and MetS risk remains poorly elucidated. The elevated serum
uric acid level observed in the MetS has been attributed to
hyperinsulinemia, since insulin reduces renal excretion of uric acid
[11]. In animal studies, hyperuricemia might induce MetS by two
mechanisms. Firstly, hyperuricemia may have a causal role in the
pathogenesis of insulin resistance. High levels of serum uric acid
inhibit endothelial nitric oxide (NO) bioavailability and insulin
requires endothelial NO to stimulate skeletal muscle glucose uptake.
Secondly, hyperuricemia induces oxidative and inflammatory changes
in adipocytes, inducing MetS in obese mice [25].
In addition to this, we previously reported a significant
relationship between serum uric acid level and anthropometric
parameters, stronger with WC than with BMI, implicating that
visceral adipose tissue is the main determinant of serum uric acid
level [26]. Furthermore, in our previous study we showed that uric
acid correlated with the majority of components of MetS, including
insulin resistance. However, these correlations were not retained in
multiple regression analysis, while only abdominal obesity (as
measured by WC), which is the most prevalent feature of MetS in the
current study, remained significant independent predictor of higher
uric acid levels. Central obesity progressively increases hepatic
and adipose tissue insulin resistance with consequent metabolic
abnormalities like impaired glucose tolerance, decreased HDL-c,
elevated triglycerides and hypertension [27]. These results suggest
an important mechanism through which obesity and hyperuricemia can
influence on higher risk of MetS and CVD.
Several limitations of our study must be emphasized. The subjected
patients were not asked to discontinue their medications, such as
antihypertensive, lipid-lowering and hypoglycemic drugs.
Furthermore, since this was a cross-sectional study, the causal
relationship between MetS and uric acid level in postmenopausal
women could not be established. Above of this, as our study was not
based on general population, selection bias might have affected the
outcome of the study. Thus larger sample size in general population
may be required to confirm the results of the present study.
CONCLUSION
To our knowledge the current study is the first one to examine
the prevalence of metabolic syndrome among postmenopausal women in
Montenegro. The obtained results are discouraging, but do not
significantly differ from other countries, which is in accordance
with the results of the increasing prevalence of MetS among
postmenopausal women in the world. It is clear that weight gain and
central obesity drives the increased prevalence of MetS in
postmenopausal women. The most prevalent feature in our study was
abdominal obesity which supported these findings. Furthermore, women
with MetS displayed higher serum uric acid levels, as compared with
those without MetS. This suggests the importance of reducing obesity
and lowering uric acid level in prevention of cardiometabolic
diseases.
Conflict of Interest Statement
The authors have declared no conflicts of interest.
REFERENCES
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell
K. Menopause and the Metabolic Syndrome: The Study of Women’s
Health Across the Nation. Arch Intern Med 2008; 168 (14):
1568-1575.
- Carr MC. The emergence of the metabolic syndrome with
menopause. J Clin Endocrinol Metab 2003; 88: 2404-2411.
- Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB.
Metabolic syndrome as a precursor of cardiovascular disease and
type 2 diabetes mellitus. Circulation 2005; 112: 3066-3072.
- Liu J, Grundy SM, Wang W, Smith SC Jr, Vega GL, Wu Z, et al.
Ten year risk of cardiovascular incidence related to diabetes,
prediabetes and the metabolic syndrome. Am Heart J 2007; 153:
552-558.
- Marjani A. A review on metabolic syndrome. J Endocrinol
Metab 2012; 2(4-5): 166-170.
- Neto JAF, Figueredo ED, Barbosa JB, Fde FB, Costa GR, Nina
VJ, et al. Metabolic syndrome and menopause: Cross-Sectional
Study in Gynecology Clinic. Bras Arch Cardiol 2010; 95(3):
339-345.
- Maury E, Brichard SM. Adipokine dysregulation, adipose
tissue inflammation and metabolic syndrome. Mol Cell Endocrinol
2010; 314: 1-16.
- Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL, Hoogwerf BJ.
Serum uric acid is an independent predictor of all‐cause
mortality in patients at high risk of cardiovascular disease: a
preventive cardiology information system (PreCIS) database
cohort study. Arthrit Rheum 2008; 58(2): 623-630.
- Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative
stress with an activation of the renin–angiotensin system in
human vascular endothelial cells as a novel mechanism of uric
acid-induced endothelial dysfunction. J Hypertens 2010; 28(6):
1234-1242.
- Nejatinamini S, Ataie-Jafari A, Qorbani M, Nikoohemat S,
Kelishadi R, Asayesh H, et al. Association between serum uric
acid level and metabolic syndrome components. J Diabetes Metab
Disord 2015; 14: 70.
- Galvan AQ, Natali AN, Baldi SI, Frascerra SI, Sanna GI,
Ciociaro DE, et al. Effect of insulin on uric acid excretion in
humans. Am J Physiol Endocrinol Metab 1995; 268(1): E1-5.
- World Medical Association declaration of Helsinki:
Recommendations guiding physicians in biomedical research
involving human subjects. JAMA 1997; 277: 925-926.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH,
Franklin BA, et al. Diagnosis and management of the metabolic
syndrome: an American Heart Association/National Heart, Lung,
and Blood Institute Scientific Statement. Circulation 2005; 112:
2735-2752.
- Hosmer D, Lemeshow S, Sturdivant RX. Applied logistic
regression, 3rd ed. New York, NY: John Wiley & Sons Inc 2013.
- Petri Nahas EA, Padoani NP, Nahas-Neto J, Orsatti FL,
Tardivo AP, Dias R. Metabolic syndrome and its associated risk
factors in Brazilian postmenopausal women. Climacteric 2009;
12(5): 431-438.
- Jouyandeh Z, Nayebzadeh F, Qorbani M, Asadi M. Metabolic
syndrome and menopause. J Diabet Metab Dis 2013; 12:1.
- Ruan X, Jin J, Hua L, Liu Y, Wang J, Liu S. The prevalence
of metabolic syndrome in Chinese postmenopausal women and the
optimum body composition
indices to predict it. Menopause 2010; 17(3): 566-570.
- Pandey S, Srinivas M, Agashe S, Joshi J, Galvankar P,
Prakasam CP, et al. Menopause and metabolic syndrome: A study of
498 urban women from western India. J Midlife Health 2010; 1:
63–69.
- Jesmin S, Islam AMS, Akter S, Islam MM, Sultana SN,
Yamaguchi N, et al. Metabolic syndrome among pre- and
post-menopausal rural women in Bangladesh: result from a
population-based study. BMC ResNotes 2013; 6(1): 157.
- International Diabetes Federation- IDF. The IDF consensus
worldwide definition of the metabolic syndrome. Brussels: IDF,
2005.
- Li Y, Chen S, Shao X, Guo J, Liu X, Liu A, et al.
Association of uric acid with metabolic syndrome in men,
premenopausal women and postmenopausal women. Int J Environ Res
Public Health 2014; 11(3): 2899-2910.
- Liu PJ, Ma F, Lou HP, Zhu YN, Chen Y. Relationship between
serum uric acid levels and metabolic syndrome in Chinese
postmenopausal women. Climacteric 2014; 17(2): 148-154.
- Dai X, Yuan J, Yao P, Yang B, Gui L, Zhang X, et al.
Association between serum uric acid and the metabolic syndrome
among a middle- and old-age Chinese population. Eur J Epidemiol
2013; 28: 669–676.
- Zurlo A, Veronese N, Giantin V, Maselli M, Zambon S, Maggi
S, et al. High serum uric acid levels increase the risk of
metabolic syndrome in elderly women: The PRO.V.A study. Nutr
Metab Cardiovas 2016; 26(1): 27-35.
- Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse
effects of the classical antioxidant uric acid in adipocytes:
NADPH oxidase-mediated oxidative/nitrosative stress. Am J
Physiol Cell Physiol 2007; 293: C584–C596.
- Klisić A, Kotur-Stevuljević J, Kavarić N, Jovanović M. The
influence of obesity on serum uric acid level in postmenopausal
women. Timočki medicinski glasnik 2016; 41(1): 20-26.
- Wang Z, and Nakayama T. Inflammation, a Link between Obesity
and Cardiovascular Disease. Mediators Inflamm 2010; 2010:
535918.
|
|
|
|


